Method
Advantages
Disadvantages
Epifluorescence
Fast
Poor axial and lateral resolution
Lower photobleaching than confocal
Limited to thin tissues
Limited control of excitation wavelength
Spinning disk confocal (SDCM)
Fast
Low excitation tissue penetration
Good axial and lateral resolution
Lower intensity and contrast than LSCM
Less photobleaching than LSCM
Laser scanning confocal (LSCM)
Best resolution and contrast in XYZ
Slow
Low excitation tissue penetration
High photobleaching
Multiphoton
Deep tissue penetration
Slow
Low photobleaching of out-of-focus planes
Poor axial resolution compared to LSCM and SDCM
Good lateral resolution
Difficult to sequentially excite different fluorophores
Single laser can excite broad range simultaneously
7.1.2.1 Epifluorescence Microscopy
Epifluorescence, or widefield, microscopy is frequently used for live imaging because of its high frame rate (up to 50 frames/s for a 512 × 512 pixel frame, depending on the camera [15]). Another advantage is that this technique can acquire transmitted light images, allowing for visualizing of tissue structure in combination with fluorescence. Unlike a confocal, all light that reaches the objective is collected, and therefore excitation intensity can be lowered to reduce photobleaching and phototoxicity. However, this leads to diminished lateral resolution due to out-of-focus light in thick tissues and explants. Epifluorescence microscopes cannot distinguish depth except via post-acquisition deconvolution [16] and so are inadequate for situations in which axial resolution is important. Epifluorescence microscopes use broad-spectrum lamps and control excitation wavelength through filter cubes. Filter wheels allow for rapid switching of excitation wavelength; however, different fluorophores must be analyzed sequentially, which decreases frame rate and may lead to poor registration between different colors in rapidly moving cells. Therefore, this technique is typically only used for samples with one or two fluorophores and only for thin tissues (<100 μm).
7.1.2.2 Confocal Microscopy
Confocal microscopy provides the best spatial resolution of the standard live cell techniques and so is often chosen for applications requiring resolution of subcellular structures. Spinning disk confocal systems can image at very high speeds (theoretically up to 2,000 fps [17], in practice highly limited by the CCD camera), making them ideal for imaging fast processes. If a very high speed is not necessary, laser scanning confocal systems can provide higher lateral and axial resolution with less noise (especially in weakly fluorescent samples), while still scanning up to 40 fps, depending on the image size. However, laser scanning confocal systems typically spend more time exciting each pixel, resulting in higher photobleaching and phototoxicity. Both types of confocal systems are limited by a small penetration depth (two to three times smaller than the maximum of a multiphoton microscope [18]) due to high scattering of visible excitation light by the sample. Unlike epifluorescence systems which use similar excitation wavelength, confocal microscopes retain high resolution in thick tissues by optically slicing with the confocal aperture. However, the aperture blocks a portion of the emitted light that reaches the detector, requiring more intense excitation and causing additional photobleaching than epifluorescence or multiphoton microscopy. The use of lasers of defined wavelengths makes it technically easier to image multiple fluorophores simultaneously, improving time resolution compared to epifluorescence microscopy. Typical imaging depths achieved by confocal microscopy are 100–200 μm.
7.1.2.3 Multiphoton Microscopy
Multiphoton microscopes excite the sample with a high-intensity, pulsed femtosecond laser with a long (infrared) wavelength to penetrate deeper into tissue with less scattering than confocal or epifluorescence microscopes (up to 500 μm) [19]. Thanks to the two-photon excitation, which only takes place in the objective’s focal point, true optical slicing is achieved so only a small volume is excited at one time, minimizing the time each point in the sample is exposed to light while retaining good spatial resolution. Because there is no need for a confocal aperture, all of the emitted light that reaches the objective is collected, thus reducing the needed intensity of the excitation. Together, these phenomena reduce photobleaching and improve the signal intensity in thick tissues, making it a common choice for in vivo imaging. Most fluorophores have a broader two-photon excitation range than single-photon excitation [20], enabling the excitation of multiple fluorophores simultaneously. A disadvantage of this is that distinguishing fluorochromes by changing excitation wavelength is generally not feasible. Multiphoton microscopy has lower resolution in all three axes than laser scanning confocal, dictated by the long excitation wavelength (typically 800–1,000 nm). The use of low-noise, high-dynamic-range photomultipliers improves sensitivity but drastically reduces acquisition speed compared to spinning disk confocal and epifluorescence microscopy. Better acquisition speeds can be realized by using a resonant scanner.
7.1.3 Choice of Multiphoton Microscopy for Studying Atherosclerosis
Atherosclerotic plaques in mouse aortas can be more than 100 μm thick [21], making two-photon excitation necessary for viewing the depth of the plaque. A low frame rate is sufficient for visualizing leukocyte movement within the arterial wall, because migration speeds are less than 1 μm/s. The low photobleaching inherent in multiphoton microscopy enables acquisition of long (~1 h) movies, which is necessary for quantifying slow migration. Multiphoton microscopy also uniquely enables the visualization of unlabeled collagen in the wall through second-harmonic generation [22]. We used multiphoton microscopy to visualize antigen presentation in the context of atherosclerosis [10].
7.2 Materials and Methods
7.2.1 Aorta Harvest
7.2.1.1 Background
Aortas can be obtained from healthy or atherosclerotic mice that have transgenically labeled leukocytes. Mice deficient in apolipoprotein E (Apoe −/−), a protein involved in lipoprotein transport, are a common model for atherosclerosis. They rapidly develop plaques when fed a high-fat western diet (WD) [23]. To study the interaction between antigen-presenting myeloid cells and T cells in atherosclerotic plaques, we used CD11c YFP mice, which express yellow fluorescent protein under the CD11c promoter [24]. This mouse has CD11b+ CD11c+ macrophages and dendritic cells in the plaque bright enough to be visualized with multiphoton microscopy. The precise relationship between YFP brightness and cell phenotype, including CD11c expression, needs to be established in each tissue and experimental setting. Non-atherosclerotic mice can be injected with 30 μg of CpG class B oligonucleotides (ODN 1826, Integrated DNA technologies), 2–3 h before sacrifice to induce myeloid cell recruitment to the aorta wall. Within atherosclerotic plaques, myeloid cells phagocytose lipids, scavenge dead cells, and secrete both pro- and anti-inflammatory cytokines [2].
7.2.1.2 Method
The mouse was killed by CO2, and 0.5–1 mL of blood was withdrawn via cardiac puncture with a 25 G needle. The internal organs were removed, and then 10 mL of PBS (containing 20 U/mL heparin) were perfused through the aorta through the heart. Using forceps and spring scissors, the fat and para-aortic lymph nodes were removed from around the artery. To maintain cell viability, it is important to disturb the wall of the aorta as little as possible, especially near the area that will be imaged, and to keep the tissue moist with PBS. The aorta was harvested, from its origin from the heart to above the renal arteries, including the branches to the innominate, left common carotid, and left subclavian arteries. Solid plaques develop first at the branch of the innominate artery from the aorta and also in the lesser curvature of the aorta. Both areas are suitable for imaging (Figs. 7.1 and 7.2). The artery was incubated overnight with T cells at 37 °C with 5 % CO2 in complete RPMI 1640 media containing 10 % FBS, 1 % pen/strep, 2 mM L-Glu, 1 % NEAA, 1 mM HEPES, and 1 mM sodium pyruvate before imaging.
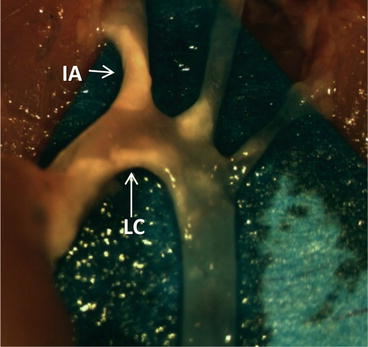
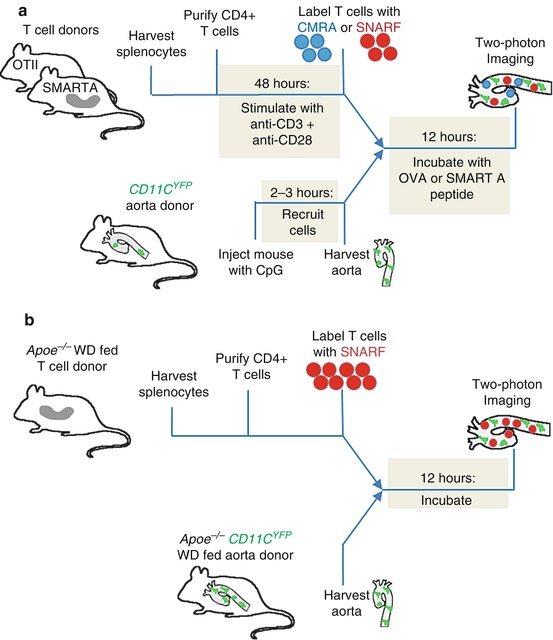

Fig. 7.1
The aorta of an Apoe −/− mouse that was fed WD for 12 weeks. After sacrifice, the aorta was perfused with PBS with 20 U/mL heparin and the artery was cleaned of fat (see Sect. 7.2.1.2) to reveal visible atherosclerotic plaque (opaque beige, white arrows). IA innominate artery, LC lesser curvature

Fig. 7.2
Schematic timeline of procedure for imaging myeloid cells presenting antigen to T cells in the aorta wall. (a) To image antigen presentation with a known, exogenous antigen, CD4+ T cells with transgenic TCRs are harvested and purified from the spleen and restimulated in vitro. YFP+ myeloid cells are recruited to the aorta with an injection of CpG, and after 2–3 h the aorta is harvested. The T cells are labeled and incubated with the aorta in the presence of exogenously added antigenic peptide, and then the tissue is imaged using two-photon microscopy. (b) To image antigen presentation in the context of atherosclerosis, the aorta of an atherosclerotic mouse with YFP+ myeloid cells is harvested. CD4+ T cells are harvested and purified from an atherosclerotic mouse. The T cells are labeled and incubated with the aorta, and then the tissue is imaged using two-photon microscopy
7.2.2 T Cell Harvest
7.2.2.1 Background
Theoretically, every T cell in a mouse may express a unique TCR that is specific for a different peptide. To investigate antigen-specific T cell activation, we used transgenic mice in which majority of CD4 T cells have a restricted TCR specificity to well-defined exogenous antigenic peptides. These mice are useful tools for studying antigen presentation because the presence of antigen can be easily controlled. We employed two different strains of mice with transgenic TCRs: OT-II, which have T cells specific for a peptide derived from ovalbumin [25], and SMARTA, which have T cells specific for a peptide derived from lymphocytic choriomeningitis virus (LCMV) [26] (see Table 7.2). Having two distinct TCR transgenic mice allowed us to image T cells with and without their cognate antigen in the same experimental setup to more accurately assess the specificity of antigen presentation in the aorta. Since neither antigen is present in a mouse, almost all T cells harvested from the transgenic mice are naïve. Naïve cells must be activated in vitro with anti-CD3 and anti-CD28 to maintain cell viability. To assay antigen presentation in the context of atherosclerosis, we also used polyclonal T cells from WD-fed Apoe −/− mice. The specific peptide that these cells recognize is unknown, but it is likely to be endogenously found in the aortas of atherosclerotic mice (see Table 7.2) [27–29]. Many T cells isolated from WD-fed atherosclerotic mice are endogenously activated, and so these cells do not require additional stimulation in vitro.
Table 7.2
Sources of T cells and the peptides they recognize
Mouse strain | Antigen protein | Peptide recognized by TCR |
|---|---|---|
OT-II | Ovalbumin | ISQAVHAAHAEINEAGR |
SMARTA | Glycoprotein P13 from LCMV | GLNGPDIYKGVYQFKSVEFD |
Apoe −/− | Unknown endogenous, possibly ApoB100, HSP60, or oxLDL | Unknown |
7.2.2.2 Method
CD4+ T cells were harvested from the spleens of atherosclerotic or TCR transgenic mice using a Robosep-negative selection kit (Stem Cell Technologies). These cells were stimulated for 48 h with 8 μg/mL anti-CD3 and 8 μg/mL anti-CD28 (eBioscience) in complete RPMI 1640 media containing 10 % FBS, 1 % pen/strep, 2 mM L-Glu, 1 % NEAA, 1 mM HEPES, and 1 mM sodium pyruvate. T cells isolated from WD-fed atherosclerotic mice were not restimulated in vitro. The cells were differentially labeled with 2.5 μM SNARF (red fluorescent carboxylic acid, acetate, succinimidyl ester, Molecular Probes) or 3 μM CMRA (CellTracker Orange, Molecular Probes) for 10 min at 37 °C. 0.5 million labeled cells were incubated with the explanted aorta in 750 μL media overnight. T cells from atherosclerotic mice were incubated with an aorta from an atherosclerotic mouse, while T cells from mice with transgenic TCRs were incubated with a healthy aorta with or without 1 μM OVA peptide or SMARTA peptide (Table 7.2).
7.2.3 Tissue Maintenance During Imaging
To visualize realistic cell motion, it is necessary to keep the tissue under physiological temperature, pH, oxygen tension, and osmolarity throughout imaging. Immediately before imaging, we secure the aorta to a coverslip by gluing the ends of the tissue with Histoacryl glue (TissueSeal LLC) or Vetbond (3 M). Tissue that touches the glue is unsuitable for imaging, so only the ends of the aorta should be glued down and only the middle used for imaging. A system (Fig. 7.3) was built to keep the tissue warm and superfused with recirculating, oxygenated media throughout the imaging procedure. A reservoir of oxygenated media was created by bubbling gas (95 % oxygen, 5 % CO2) through ~25 mL of complete RPMI 1640 without phenol red, with 2 mM L-glutamine, 1 % pen/strep, and 1 % FBS, in a 50 mL conical tube. The sample glued to the coverslip was placed in a 60 mm dish with the same solution. A peristaltic pump (Harvard Apparatus, MPII) circulated media at approximately 10 mL/min from the reservoir through an in-line solution heater (Warner Instruments, SF-28) to the dish. The dish was heated by a warmer (Warner Instruments, QE-2 Quick Exchange Platform), and both this and the in-line heater were controlled by a dual feedback control unit (Warner Instruments, TC-334B) that maintained the liquid near the tissue at 37 °C. The peristaltic pump also returned the media from the dish to the reservoir so that the media constantly recirculate. The inlet and outlet to the dish must be as far as possible from the tissue to not disturb the imaging.
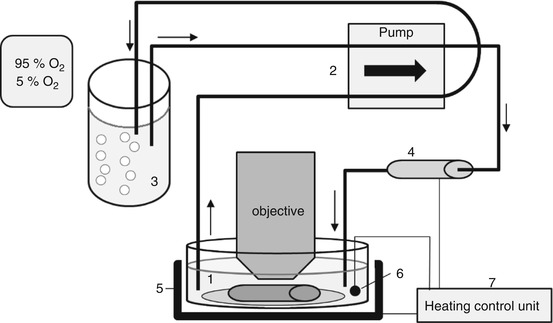

Fig. 7.3
Schematic diagram of recirculating superfusion and heating system to maintain aorta under physiological conditions during imaging. An aorta explant is incubated with T cells for 12 h to allow the T cells to migrate into the tissue. The artery is glued to a coverslip, and placed in a dish with complete RPMI 1,640 without phenol red, with 2 mM L-glutamine, 1 % pen/strep, and 1 % FBS. (1) A peristaltic pump (2) circulates the media from an oxygenated reservoir, (3) through an in-line solution heater (4), to the dish and back to the reservoir. The sample is warmed by a dish-warmer (5). The media temperature is monitored by a thermometer (6), and a control unit (7) regulates both heaters to maintain a solution temperature of 37 °C. Arrows show the direction of fluid flow
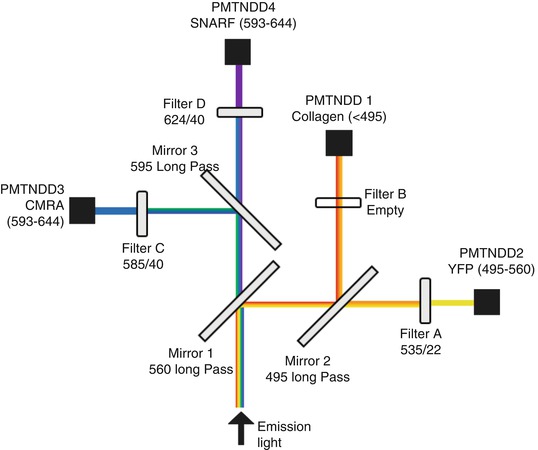
7.2.4 Microscopy and Hardware
Imaging was performed on a Leica TCS SP5 multiphoton system. This system utilizes a DM 6000 upright microscope with a 20× (NA = 0.95) water-dipping objective (Olympus, XLUMPLFL), attached to a piezo-controlled objective holder (Piezosystem Jena, NV 40/1 CLE and MIPOS 500 SG) to set the focal plane. A Coherent Chameleon Ultra II Ti:sapphire-pulsed femtosecond laser excites the sample with wavelengths between 680 nm and 1,080 nm. Emitted light is split through a series of three dichroic mirrors and four filters up to four non-descanned photomultiplier tube detectors (PMT-NDD) (Fig. 7.4). The optical path to the detectors does not return through the scanning mirror, improving the sensitivity of detection. Laser scanning is accomplished with either a conventional or resonant scanner, depending on the needs of the experiment. The resonant scanner scans each line faster than the conventional scanner (8,000 lines/s compared to 200–1,400 lines/s). The speed can be effectively doubled in the bidirectional mode which allows for horizontal scanning in both directions, though this can lead to interlacing errors. The pixel dwell time of the resonant mode is shorter, which leads to a higher frame rate and less photobleaching, but more noise. Line or frame averaging can reduce this noise and may still allow for better time resolution than with a conventional scanner.