, Amit Aggarwal2, Javin Schefflein3 and A. Orlando Ortiz4
(1)
Division of Neuroradiology, Neuroradiology Fellowship, Mount Sinai Hospital, New York, NY, USA
(2)
Neuroradiology Fellowship, Mount Sinai Hospital, New York, NY, USA
(3)
Department of Radiology, Mount Sinai Medical Center, New York, NY, USA
(4)
Department of Radiology, Winthrop-University Hospital, Mineola, NY, USA
Keywords
AnatomyLumbar spineBiopsy diskBiopsy paraspinal soft tissuesBiopsy vertebraeLumbar spine biopsy coaxial techniqueImaging guidance computed tomographyImaging guidance fluoroscopyPercutaneous spine biopsy complicationsPercutaneous spine biopsy indicationsPercutaneous spine biopsy contraindicationsSpine biopsy lumbar spineLearning Objectives
- 1.
To learn the pertinent radiologic anatomy, including bony, neural, and vascular anatomy, as it relates to image-guided lumbar spine biopsy
- 2.
To review the most common indications and contraindications for image-guided lumbar spine biopsy
- 3.
To review different approaches and techniques when planning image-guided coaxial lumbar spine biopsy
6.1 Introduction
Of all the image-guided percutaneous spine biopsy procedures that are performed along the spinal axis, lumbar spine biopsy is the most frequently performed of these procedures.
Compared to the cervical spine, percutaneous biopsy of the lumbar spine is relatively safer and technically less difficult. The thicker lumbar pedicles and larger surface area for initial needle insertion allow ease of navigation around adjacent critical nerves and vasculature. Numerous pathologic entities, including infectious and neoplastic processes, can originate from or spread to the lumbar spine and paraspinal tissues. A study of 410 biopsied lumbar spine lesions found metastatic breast and lung cancer to be the most common etiologies found in women (28% and 7%, respectively), while metastatic lung and prostate cancer were found to be the predominant etiologies in men (12% and 7%, respectively) (Lis et al. 2004). Image-guided percutaneous lumbar spine biopsy is generally useful, as the vast majority of procedures yield an adequate specimen for diagnosis (Kornblum et al. 1998). Due to the variety of pathologies affecting the lumbar spine, the diagnostic yield of a biopsy sample varies depending upon the cause of disease as well as on the internal architecture of the lesion. Lumbar spine biopsy for primary and metastatic tumors has an accuracy of approximately 90%. The reported accuracy of spine biopsy for infection is less accurate, only providing a diagnosis around 50% of attempted biopsies (Hau et al. 2002). In spite of the high rate of sample adequacy, the most frequent adverse outcome remains nondiagnostic sampling, especially for the evaluation of spine infection. Image-guided percutaneous lumbar spine biopsy possesses the lowest rate of diagnostic utility in lesions that contain a large necrotic component, diffuse vascularity, or are densely blastic (Sundaresan et al. 2004; Wu et al. 2008). Sclerotic lesions present a particular challenge compared to their lytic counterparts due to the technical difficulty denser tissue presents when extracting a sample. In addition, clinical scenarios in which the likelihood of neoplastic or infectious pathology is low, but there is an overlap in the imaging findings between neoplastic and degenerative pathologic processes or between infectious and inflammatory or traumatic processes, raise the suspicion for neoplasm or infection just enough that a biopsy is requested. For example, in an older patient, the presence of extensive degenerative changes of the spine, and a concomitant diagnosis of metastatic disease, may lead to a false suspicion of sclerotic metastases (Ghelman et al. 1991). For similar reasons, differentiating pathologic from benign osteoporotic compression fractures in an elderly patient population presents an ongoing challenge to clinicians.
Over the past several decades, due to a combination of progressive advancements in radiology imaging equipment, image-guiding technology as well as the instruments for performing these procedures, image-guided percutaneous biopsy techniques have grown in use and utility. Despite open biopsy remaining the ultimate procedure for diagnosis, percutaneous spine biopsy has become the preferred method at most institutions around the world (Hau et al. 2002). CT- and fluoroscopic-guided biopsy provides numerous advantages over open biopsy, the most notable of which is a lower morbidity (Chooi et al. 2007). Percutaneous access affords numerous benefits over open surgical techniques, including lower rates of postoperative wound infection, a lower incidence of post-biopsy pathologic fracture, and avoidance of complications from general anesthesia (Schajowicz and Derqui 1968; Murphy 1983). Furthermore, image-guided spine biopsy serves as a cost-effective diagnostic tool, due to the shorter procedure time compared with open techniques and the shorter post-procedure recovery time (Schajowicz and Derqui 1968; Murphy 1983; Peh 2006). Post-biopsy observation varies by institution, but, generally, lasts no longer than 3 h, whereas open techniques may require an overnight hospital stay (Lis et al. 2004). Percutaneous lumbar spine biopsy is an invaluable tool in establishing a diagnosis and guiding subsequent disease-specific treatment. This chapter aims to elucidate the various techniques of fluoroscopic- and CT-guided lumbar spine biopsy as well as provide an overview of different indications and complications that the operator should consider prior to performing the procedure.
6.2 Anatomic Considerations
The lumbar spine typically consists of five lumbar vertebrae and their intervening intervertebral disks (Fig. 6.1). Although biopsy at the level of the lumbar spine is often technically less demanding compared to other locations in the spine, it remains crucial to consider the anatomical structures surrounding the lumbar vertebrae and intervertebral disks. Image-guided percutaneous lumbar spine biopsies are almost universally performed from a posterior approach. The operator must guide the needle through the posterior paraspinal musculature including the erector spinae and multifidus or quadratus lumborum muscles, in order to access the vertebral body and/or paravertebral soft tissues. Often a transpedicular approach is selected for access to a vertebral body lesion as the pedicle size within the lumbar vertebra can easily accommodate most commercially available biopsy needle systems. Access via the pedicle, if possible, can serve as a safe conduit into the vertebral body (Chooi et al. 2007). Care must be taken to ensure that the medial border of the pedicle is not breached prior to entering the posterior vertebral body in order to avoid inadvertent entrance into the spinal canal. Although the location of the needle tip must be monitored during a transpedicular approach, there is reduced risk to adjacent soft tissues, nerves, or vasculature once the needle passes within the safe channel provided by the pedicle. An alternate approach for biopsy, however, may be necessary depending upon the location of the lesion within the vertebral body. For example, smaller posterior median lesions may not be accessible via a transpedicular route. The intervertebral disk and the paraspinal soft tissues, likewise, are usually not accessed via a transpedicular approach. Posterolateral or extrapedicular approaches are optional trajectories, and an active awareness of the anatomic location of neural and vascular structures is critical toward reducing the risk of injury with these approaches. The lumbar arteries arise from the aorta and run along the equatorial or midportion of the vertebral body, coursing posteriorly, where they enter the neural foramina bilaterally. These arteries give rise to both vertebral nutrient arteries which supply blood to the vertebrae and radiculomedullary arteries which can contribute to the blood supply of the spinal cord and cauda equina. Visualization of these vessels is often difficult during lumbar spine biopsy, especially since the procedure is performed without the use of intravascular contrast agents when using imaging guidance. It is nevertheless important to consider and be aware of these vascular structures when using a posterolateral approach.


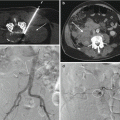
Fig. 6.1
CT anatomy of the lumbar spine. Midline reformatted sagittal CT image (a) in bone window algorithm shows five lumbar vertebral bodies (L1–L5) and their corresponding spinous processes (arrows) and the intervertebral disks (curved arrows); note the orientation of the most caudal disk space (lower curved arrow). Reformatted left parasagittal CT image (b) in bone window algorithm shows the vertebral pedicle (curved arrow) and its relationship to the posterior vertebral body and to the neural foramen (dashed oval); the aorta is partially visualized (arrow). Close-up of same image (c) in soft tissue algorithm shows the L2 dorsal root ganglion (curved arrow) within the neural foramen at the L2–L3 level; the proximity of posterior disk (arrow) to the nerve is also noted. Contrast-enhanced axial CT image (d) in soft tissue algorithm shows the vertebral body (VB), pedicle (P), transverse process (TP), lamina (L), and spinous process (sp). The erector spinae and multifidus muscles lie posterior to the posterior elements, and the psoas muscle lies lateral to the vertebral body. The lumbar artery (arrows) is segmentally visualized along the midpole of the vertebral body. The aorta (A) and inferior vena cava (IVC) lie anterior to the vertebral body, and the kidneys are seen laterally. The bowel is seen anterior to the aorta and inferior vena cava. Contrast-enhanced axial CT image (e) in soft tissue algorithm at the level of the intervertebral disk shows the dorsal root ganglia (arrows) and the base of the spinous process (sp). Axial CT image (f) in bone window algorithm shows the trabecular structure of the vertebral body (VB) and a small central focal defect within the posterior vertebral body cortex that corresponds to the basivertebral plexus (curved arrow). The spinal canal (Sc) is bordered anteriorly by the posterior vertebral cortex, laterally by the pedicles and posteriorly by the laminae. The laminae join to support the spinous process (sp). The posterior surface of the pedicle (arrow) lies medial to the transverse process and is the access point for a transpedicular approach. Axial CT image (g) in bone window algorithm at the level of an upper lumbar facet joint (large arrow) shows the more lateral superior articular facet (small arrow) that corresponds to the vertebra shown and the more medial inferior articular facet (curved arrow) of the vertebra above
Often a transpedicular approach is selected for access to a vertebral body lesion as the pedicle size within the lumbar vertebra can easily accommodate most commercially available biopsy needle systems.
The spinal cord terminates as the conus medullaris usually at the L1–L2 level of the spinal canal. This critical structure must be accounted for when considering lumbar spine biopsy procedures within the upper lumbar spine. The cauda equina, a constellation of sensory and motor nerve roots, arises from the conus medullaris. These nerve roots are named for the vertebra bordering the superior portion of the neural foramen through which they pass (e.g., the L2–L3 neural foramen contains the L2 nerve root). These nerves enter the proximal neural foramina bilaterally above the level of the disk space at their respective intervertebral level and course along the superior portion of the foramen. As the nerve roots exit the foramen, they course inferiorly. The L1–L4 nerve roots join to form the lumbar plexus which runs along and within the psoas musculature. The operator must consider the location of the nerve roots when performing a posterolateral approach. These approaches are frequently performed with CT guidance and therefore allow direct but segmental visualization of the nerve with respect to needle trajectory.
CT guidance can offer more direct visualization of anatomic structures related to the lumbar spine which may be particularly helpful when using a posterolateral approach.
The aorta runs along the anterior margin of the lumbar spine, almost always on the left side; the inferior vena cava is located on the right side (Fig. 6.1). A lesion may occasionally extend to and penetrate the anterior cortex of the vertebral body. Identification of the lesion’s extension with respect to the aorta and inferior vena cava is important in preventing potential injury to these vital structures. Injury to the aorta is rare, as most lesions are confined to the vertebral bodies. It may be appropriate to utilize CT guidance in these cases to ensure proper visualization of the aorta and inferior vena cava in relation to the biopsy needle. If fluoroscopic guidance is considered, the use of lateral imaging is necessary during advancement of the biopsy needle to prevent penetration of the anterior cortex of the vertebral body or the anterior aspect of the intervertebral disk. The lumbar sympathetic plexus is located bilaterally along the anterior and lateral aspect of the lumbar vertebral column. Fortunately, no significant injuries to this structure with lumbar spine biopsy have been reported. The other critical organs, however remote, that must always be considered when performing lumbar spine biopsy include the inferior aspects of the lungs and pleura, the kidneys, and the bowel.
Once a request for percutaneous image-guided lumbar spine biopsy has been received, all available and pertinent imaging studies must be reviewed and a planned needle trajectory formulated bearing in mind the aforementioned anatomic structures. Consideration of needle approach will be required to ensure proper sampling of the lesion within the vertebral body, intervertebral disk, or paraspinal soft tissue. All approaches rely on a careful review of the available imaging examinations and the assessment of anatomic structures, lesion location, and lesion size in order to ensure that the optimal needle trajectory is chosen.
Knowledge of the critical anatomy of the lumbar spine is extremely important in planning needle trajectory for image-guided percutaneous lumbar spine biopsy and for avoiding complications.
6.3 Indications
Image-guided percutaneous biopsy of the lumbar spine allows access to the vertebral body, posterior elements, intervertebral disks, as well as surrounding soft tissues. Requests for lumbar spine biopsies are most frequently a result of imaging (i.e., CT, MRI, PET-CT, bone scan) that shows the possible presence of a neoplastic or infectious process (Peh 2003; Hodge 1997). A list of most common indications for image-guided percutaneous lumbar spine biopsy is provided in Table 6.1. Histopathologic identification of malignancy plays an important role in the management of newly diagnosed malignancy, modification of current treatment, and assessment of prognosis in metastatic disease of a known primary malignancy (Sundaresan et al. 2004; Herkowitz and Wesolowski 1986). In particular, tissue identification of specific etiologies of malignancy may change oncologic management – a patient previously planned for surgery may be discovered to have a plasmacytoma or lymphoma involving the spinal canal and thus would benefit from other treatments such as chemotherapy or radiation. Even after treatment, patients many times require lumbar biopsy to exclude disease recurrence when a new lesion is identified on an imaging study (Peh 2003; Hodge 1997). More recent advances in medical therapy have reinforced the importance of biopsy, such as evaluating active lesions demonstrated on PET imaging. Emerging concepts such as tailoring specific treatment regimens to the inherent biologic heterogeneity of a neoplasm requires biopsy for accurate tissue sampling and subsequent genetic analysis (Talac and Mclain 2009).
Table 6.1
Indications for image-guided percutaneous lumbar spine biopsy
1. Infection |
Spondylitis-diskitis |
Paraspinal abscess |
2. Neoplasm |
Primary osseous neoplasm |
Evaluation of solitary bone lesion |
Secondary osseous neoplasm |
Osseous metastatic disease or involvement by systemic malignancy |
Diffuse marrow replacement process |
Evaluation of neoplastic lesions with diffusion restriction or FDG-PET avidity posttreatment to assess for treatment response |
Paraspinal soft tissue mass |
Pathologic vertebral body compression fracture |
3. Pretreatment (including the above categories) |
Tissue characterization prior to treatment initiation |
Lumbar spine biopsy may be requested in patients who were initially considered to have benign disease, but subsequent clinical evaluation suggests otherwise. For example, in patients with back pain secondary to what is initially thought to be an osteoporotic vertebral compression fracture, when symptoms worsen or concerning imaging characteristics such as progressive tumor growth or pathologic abnormality emerge, tissue sampling may be beneficial to exclude malignancy (Herkowitz and Wesolowski 1986). Additionally, equivocal imaging findings or patient anxiety from uncertainty may result in a request for image-guided biopsy. Another common indication for lumbar spine biopsy is for definitive diagnosis of vertebral osteomyelitis and/or diskitis (Peh 2003; Hodge 1997; Herkowitz and Wesolowski 1986). Occasionally, the clinical presentation raises suspicion for spinal infection, which can be confirmed with MR imaging of the spine. In this setting, lumbar spine biopsy plays an important role in identification of the infectious organism, which enables antimicrobial therapy to be tailored specific to the infectious pathogen. Alternatively, both imaging and clinical findings may not be specific for spinal infection, which then requires biopsy with surgical pathology evaluation of core tissue from the disk and/or vertebral body for diagnostic confirmation or exclusion.
6.4 Contraindications
Image-guided percutaneous lumbar spine biopsy is a relatively safe procedure with few contraindications (Table 6.2). A major contraindication, however, is uncorrected coagulopathy (Santiago et al. 2014). This is often the result of anticoagulant therapy, but may also be seen in patients who have intrinsic coagulopathy due to underlying malignancy or other disease states. When possible, it is important to hold anticoagulation therapy prior to procedure to reduce risk of hemorrhage (refer to the Chap. 2). Consultation with the provider managing this therapy is often necessary to ensure that holding this medication does not produce additional risk to the patient due to a thromboembolic event. In patients with intrinsic coagulopathy, it may be necessary to infuse platelets or administer vitamin K prior to the procedure, such as when platelet counts fall below 50,000/mcL (Peh 2006). Again, the operator should discuss the appropriate management of the coagulopathy with the responsible patient care provider(s). Occasionally, consultation with a hematologist may provide additional insight into the appropriate management.
Table 6.2
Contraindications to image-guided percutaneous lumbar spine biopsy
Absolute |
Uncorrected coagulopathy |
Untreated infection in patient with suspicious mass lesion |
Relative |
Patient factors |
Combative or uncooperative patient |
Clinically unstable patient |
Lesion type |
Vascular lesion |
Probable benign lesion |
Lesion size |
Discretion must be exercised with smaller lesions (< 5 mm in diameter) |
Limited or no specimen yield may result in false negative biopsy |
Lesion location |
Defer biopsy for lesions located adjacent to critical structures or inaccessible locations |
Preexisting infection at the skin site, such as a cellulitis or a decubitus ulcer, can sometimes occur near the intended area for possible percutaneous biopsy. Infection at the skin site or within the soft tissues surrounding a tumor can be considered a contraindication to percutaneous biopsy (Peh 2003; Hodge 1997; Ghelman 1998). Although unlikely, the spread of the infection into deep soft tissues, tumor, or within the vertebral body can occur if a soft tissue infection, such as a cellulitis, is not treated prior to biopsy. Consultation with an infectious disease specialist may be necessary to optimize antibiotic therapy and provide medical clearance prior to biopsy. Patients who are uncooperative or unstable are not candidates for image-guided lumbar spine biopsy (Peh 2006). If a patient is clinically unstable, it is prudent to wait until the patient is medically stabilized as well as to consult with the patient’s clinical providers in order to assess for the urgency and clinical need for a biopsy. With respect to uncooperative patients, after discussion with the appropriate patient representative and requesting provider, a clinically necessary lumbar spine biopsy can be performed under general anesthesia or monitored anesthesia care. The risk and benefits of the anesthesia and the need for tissue diagnosis must be carefully assessed in order to appropriately triage candidates for the procedure. The type of lesion may also influence whether or not a biopsy gets performed. Hypervascular lesions may dissuade an operator for fear of a hemorrhagic event. The operator should try to avoid performing biopsy procedures in cases where the radiographic features are highly suggestive or pathognomonic of a benign lesion (Figs. 6.2 and 6.3). Very small (less than 5 mm diameter) lesions may not be amenable to biopsy; it just may not be possible to obtain tissue. Lesions, especially small lesions, that are located near critical structures such as the spinal cord, lung, or aorta may also not be amenable to percutaneous biopsy.



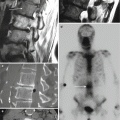
Fig. 6.2
Axial CT image shows posterolateral approach with bone biopsy needle (arrow) for biopsy of a round sclerotic lesion with a lucent center at the vertebral endplate. This is a Schmorl’s node and a biopsy was not necessary

Fig. 6.3
A 20-year-old male with chronic low back pain. T1-weighted axial image (a) shows a hypointense lesion within the pedicle (arrow) and a linear hypointense defect within the opposite pedicle (curved arrow). The clinicians and family insisted upon a biopsy procedure for what is obviously a stress fracture. Axial CT image (b) in bone window algorithm shows sclerosis in the right pedicle (arrow) and a fracture line (curved arrow) in the left pedicle. Axial CT image (c) shows biopsy of the sclerotic pedicle with a bone needle (arrow). The biopsy was negative
6.5 Risks and Complications Associated with Lumbar Spine Biopsy and How to Minimize Them
It is important for the operator to be aware of the potential complications that have been associated with lumbar spine biopsy in order to help reduce the overall risk to the patient during this procedure (Ortiz et al. 2010) (Table 6.3). Complications from image-guided percutaneous lumbar spine biopsy are relatively uncommon, with a reported rate of less than 1–3% (Tehranzadeh et al. 2007). Although image-guided lumbar spine biopsy is safely performed on a routine basis, operators must be aware of potential complications in order to first prevent and avoid them and, second, to acutely manage such situations in order to avoid further injury to the patient. Lumbar spine biopsy complications can be divided into acute and delayed or late complications (Tehranzadeh et al. 2007). Acute complications after lumbar spine biopsy include subcutaneous hemorrhage or hematoma formation, hemorrhage from biopsy of hypervascular lesions, neurologic injury, dural puncture, and vertebral fracture. Specifically, renal cell carcinoma and thyroid carcinoma are well-known examples of hypervascular tumors that are prone to hemorrhage when biopsied (Talac and McLain 2009). An acute or even subacute complication that is often overlooked by operators is the occurrence of a thromboembolic event (myocardial ischemia in a patient with coronary artery stents or stroke in a patient with atrial fibrillation) in a patient when anticoagulant or antiplatelet medication has been transiently discontinued. Late complications, which can arise weeks to months after the spine biopsy procedure, include infection and tumor seeding along the needle tract. Risk of infection is low in the setting of percutaneous biopsy when performed with appropriate sterile preparation of the biopsy entry/access site. In one study, no post-procedural infections were reported out of 94 CT-guided spine biopsy cases (Olscamp et al. 1997). Needle tract seeding by tumor is also a late complication and is rare when utilizing specific coaxial techniques and the smaller gauge needle sizes that are typically used for spine biopsy (Saghieh et al. 2010; Davies et al. 1993).
Table 6.3
Percutaneous lumbar spine biopsy: potential risks and complications
Tissue injury |
Vascular injury |
Neural injury |
Pneumothorax |
Hemorrhage Superficial or subcutaneous Deep – hemorrhage into a tumor and/or spinal canal can result in acute neurologic changes, or retroperitoneal hemorrhage can result in hypotension or severe pain |
Infection (superficial or deep) in those cases being performed to assess for neoplasm |
Inappropriate needle placement Breach of the anterior vertebral body or medial pedicle cortex Needle placement within the spinal canal Wrong level |
Inadequate tissue sampling |
Technical failure – biopsy system failure, lost specimen |
Tumor seeding along the biopsy tract |
Radiation exposure |
Anesthesia complications Aspiration, airway compromise, respiratory depression |
Thromboembolic events in patients with reversed anticoagulation/antiplatelet therapy |
Hemorrhage can occur with any invasive procedure, and a minimal amount is often unavoidable. Therefore, correction of coagulopathy is important to help reduce the risk of significant hemorrhage during lumbar spine biopsy. As previously mentioned, a coaxial approach minimizes the need for multiple passes through the skin, subcutaneous soft tissues, and muscles when accessing the vertebral body or paravertebral soft tissues. Reduced manipulation of the adjacent soft tissue results in reduced risk of injury to vascular structures. In the lumbar spine, the lumbar arteries typically pass along the equatorial plane of the vertebral body and traverse the anterior margin of the neural foramen to enter the posterior vertebral body as the nutrient supply to the vertebral body (Fig. 6.1). Although there are multiple periosteal arteries that originate along the course of the lumbar artery, it is not usually necessary to identify these vessels since access into the vertebral body is transpedicular or parapedicular, avoiding injury to these vascular structures.
Biopsy of hypervascular tumors can result in excessive bleeding. Typically, waiting 5–10 min with the stylet placed in the introducer needle when using a coaxial needle system will result in hemostasis. Occasionally, it may be necessary to inject Gelfoam or Surgifoam into the introducer needle along the biopsy tract to achieve hemostasis (Talac and McLain 2009). The use of a smaller needle in these types of lesions also can reduce the risk of hemorrhage. An alternative to core needle biopsy of suspected hypervascular lesions is fine-needle aspiration. This technique allows for the placement of a small needle, typically 25 or 27 gauge, and can often yield enough cellular tissue to establish a histologic diagnosis.
Injury to the lumbar nerve roots, as well as the thecal sac (dura mater), can be avoided with the use of CT guidance. Visualization of these structures in relation to the advancing needle tip can allow the operator to modify needle trajectory or establish a new path of needle placement if there is concern for injury to neural structures. It is important to be able to identify important bony landmarks when performing spine biopsy under fluoroscopic guidance as direct visualization of neural structures is not possible with this imaging modality. Specifically, when using a transpedicular approach, it is necessary to ensure that the biopsy needle does not penetrate the medial margin of the pedicle as seen on the anterior-posterior fluoroscopic projection prior to reaching the posterior margin of the vertebral body as seen on the simultaneous lateral fluoroscopic projection. Confirmation of this precise needle positioning will ensure that the needle does not penetrate the medial border of the pedicle and advances into the spinal canal (Fig. 6.4).


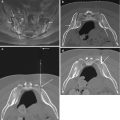
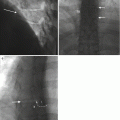
Fig. 6.4
Initial needle positioning for the transpedicular approach. Oblique and lateral fluoroscopic images (a) obtained simultaneously with a bone needle docked on the posterior surface of the pedicle (arrows). The oblique orientation shows the needle tip within the “eye of the Scotty dog” (arrow). Frontal and lateral fluoroscopic images (b) obtained simultaneously show advancement of the bone needle through the pedicle to the margin of the posterior vertebral body as shown on the lateral image (arrow). It is very important to observe that the needle tip has not crossed the medial pedicle cortex (arrow) as shown on the frontal projection (if it had, then the needle tip would be in the spinal canal!). A photograph of a plastic see-through vertebral body model (c) from an overhead view with a bone needle (curved arrow) inserted to the same position as in (b) shows the tip of the needle (large arrow) at the junction of the anterior pedicle and posterior vertebral body. Note that the needle tip has not crossed the medial pedicle cortex (dashed arrow); compare to the frontal fluoroscopic image in b. A photograph of the model from a lateral view (d) with the needle in the same position shows the needle tip at the pedicle-vertebral body junction (arrow); compare to the lateral fluoroscopic image in b
6.6 Imaging Guidance
The most commonly utilized modalities for image-guided percutaneous lumbar spine biopsies are CT, CT fluoroscopy, and fluoroscopy. A meta-analysis of 25 studies revealed accuracy rates of 90.2% and 88.1% for CT- and fluoroscopic-guided biopsies, respectively, when compared to the subsequent clinical confirmation of the diagnosis (Nourbakhsh et al. 2008). The use of other modalities, such as MRI and ultrasound, is limited in the setting of lumbar spine biopsies. It is often challenging to use MRI for imaging guidance due to limited scanner availability, procedure time, and the need for specialized MRI-compatible biopsy equipment. Additionally, patients with certain types of implants (e.g., certain aneurysm clips and non-MRI conditional pacemakers) cannot be placed in MR scanners. Ultrasound can be used for superficial soft tissue biopsy or aspiration of subcutaneous fluid collections, but cannot be reliably utilized for deep bone lesions due to the significant shadowing that occurs with cortical bone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree