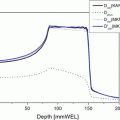
Fig. 21.1
The treatment system. (A) The tumor shadow and metallic markers are shown by white arrows. (B) Patients receive the carbon ion beams from four directions, as indicated by the arrows. (C) A respiratory-gated irradiation system using infrared from an LED placed on the body surface
21.2.3 Immobilization
The immobilization devices consist of polyurethane fixtures and thermoplastic plates. The fixtures and plates are personalized for each patient before CT scanning for treatment planning. The patient is usually treated in the supine position. If the tumor is in the posterior lung, the patient assumes a prone position.
Irradiation is typically administered from four directions. As the beam lines are fixed in vertical and horizontal directions, the other two directions are achieved by tilting the patient to the left or right (Fig. 21.1).
21.2.4 Respiratory Gating
CT images for treatment planning are taken in synchronization with the respiratory motion. Because the displacement of a tumor is generally lowest at the end of the expiratory phase, this timing is applied for the actual irradiation. The respiratory sensing system uses a position-sensitive detector (PSD) as a camera, along with an infrared light-emitting diode (LED). The LED is attached to the patient’s body around the chest wall, and the light spot from the LED is focused on the PSD through a lens system [20]. A change in position is amplified by the zoom lens of the camera. The analog signals of the PSD are directly proportional to the spot position, without the need for a software program to calculate the position. The camera is typically mounted on the treatment couch at the feet of the patient, where it does not disturb the irradiation and does not interfere with the patient’s fixation device. Setup of the respiratory sensor takes less than 30 s (Fig. 21.1). Prompt starting and stopping of the beam extraction according to the gate signal are achieved by a special extraction method that provides an efficiency of more than 85 % of that of the standard extraction at the heavy ion medical accelerator in Chiba (HIMAC)[20, 21].
21.2.5 Irradiation
For patient positioning, fluoroscopic images are used, along with the superposition of the respiration waveform. Each treatment room of the HIMAC has a pair of orthogonal fluoroscopic devices. Fluoroscopic images of the patient in the optimal position are digitized and transferred to the positioning computer. They are displayed on the computer monitor screen, together with reference images, such as simulation images or digital reconstruction radiography, which are calculated based on the planning CT images. Fluoroscopy is performed from the beam’s eye view. The patient’s respiration waveform and the gate signal are also superimposed on the TV screen. The treatment couch is then moved to the matching position until the largest deviation from the field edge and the isocenter position is less than 2 mm. The whole procedure, including irradiation, takes about 20–30 min.
21.2.6 Follow-Up
Most patients underwent clinical examinations for follow-up, and CT scans of the thorax were carried out at our institute. Patients in whom follow-up testing could not be carried out until completion underwent periodic CT scanning at another institute. The clinical outcomes of all patients have been confirmed.
The first follow-up examinations were performed 4 weeks after C-ion RT and were repeated every 3–4 months. It is difficult to distinguish the changes in normal tissues due to radiation from tumor regrowth. We therefore defined transitorily enlarged densities observed after approximately 3 months as locally controlled tumors. Local recurrence was defined by an enlarging tendency of the tumor, as well as based on the findings of CT images, PET scans, tumor marker levels, and biopsy results.
21.3 Stage I Non-small Cell Lung Cancer
21.3.1 Stage I Peripheral Type Tumors
21.3.1.1 Treatment Planning
The targets are typically irradiated from four oblique directions without prophylactic elective nodal irradiation. A margin greater than 10 mm is set outside the gross target volume (GTV) to determine the clinical target volume (CTV). Spicular formations and pleural indentations are included in the CTV where possible. An internal margin (IM) is set outside the CTV in order to allow for target motion during gating. The planning target volume (PTV) is defined as the CTV plus IM. Three-dimensional treatment planning is performed using the HIPLAN software program, which was developed at the NIRS [22]. The IM is determined by extending the target margin in the head and tail directions by a width of 5 mm, which has resulted in the successful prevention of marginal recurrences caused by respiratory movement [23].
Compared with the pulmonary damage reported in stereotactic radiotherapy for stage I NSCLC [24, 25], the incidence and severity of the damage in our patients seem to be low. These mild adverse effects for the lung were achieved as a result of the small irradiated volume (V20 #9802 T1 (n = 30) mean 5.5 % (2.3–11.6), T2 (n = 21) mean 7.6 % (2.6–13.9), #0001 T1 (n = 41) mean 4.8 % (1.1–13.2), T2 (n = 39) mean 6.4 % (1.0–12.3)) achieved with the excellent dose distribution provided by the carbon ion beams due to the formation of a Bragg peak, which is in contrast to the results when X-rays are used as the permeating beam (Fig. 21.2).
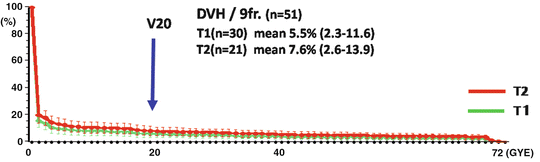

Fig. 21.2
The DVH of the phase II study (9802)
21.3.1.2 A Representative Case of Single Fraction C-Ion RT
An 83-year-old Japanese male was referred to the NIRS hospital with a diagnosis of primary lung cancer, of which the initial stage was T2N0M0 and the pathology was squamous cell carcinoma. The CT scan demonstrated that the tumor was located in the upper lobe (S3) of his right lung (Fig. 21.3).
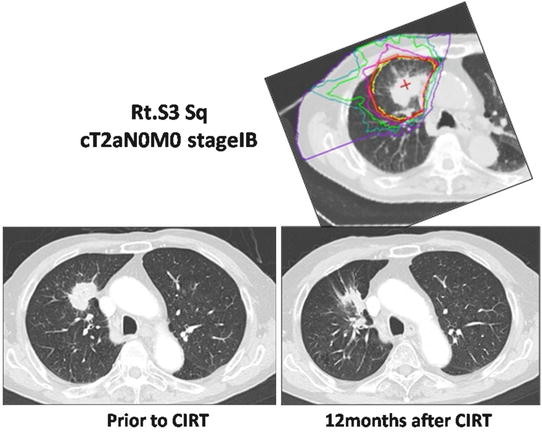

Fig. 21.3
The dose distribution of the planning CT and CT images pre- and postirradiation
CIRT was performed with four ports in four directions, and the total dose was 46.0 GyE in a single fraction. Twelve months later, the tumor demonstrated by the follow-up CT was noted to have decreased in size. The patient is still alive without any recurrence or metastasis 3 years after the CIRT.
21.3.1.3 Results
The local control rate for all patients (trial #9802 and #0001) was 91.5 %, and the rates for T1 and T2 tumors were 96.3 and 84.7 %, respectively. While there was a significant difference (p = 0.0156) in the control rates between T1 and T2 tumors, there was no significant difference (p = 0.1516) between squamous cell carcinomas and non-squamous cell carcinomas. The 5-year cause-specific survival rate was 67.0 % (IA: 84.4 %, IB: 43.7 %), and the overall survival was 45.3 % (IA: 53.9 %, 1B: 34.2 %). No adverse effects greater than grade 2 occurred in the lung in any of the patients.
In a single fractionation trial, the 3-year local control rate for 151 patients was 83.1 %, and the control rates for T1 and T2 tumors were 88.5 and 75.0 %, respectively. The overall survival rate was 75.5 %. No adverse effects greater than grade 2 occurred in the lung in any of these patients.
21.3.2 Stage I Central-Type Tumors
21.3.2.1 Treatment Planning
When treating lesions located near the lung hilus, there are concerns about the potential for serious damage to the pulmonary function resulting from bronchial stenosis [26]. During the treatment of peripheral lung tumors in our studies, we observed very little loss of pulmonary function after the C-ion RT [27]. However, any reduction of the pulmonary function is an adverse effect that can significantly impair the quality of life. We have therefore been making an extensive effort to minimize the risk of severe bronchial toxicity during the treatment of central tumors. Adequate target coverage (local control) and the sparing of the airway (unrelated segment bronchus) are necessary. For the airway sparing, using a small CTV (cutting off the bronchus from a large CTV) is important.
21.3.2.2 Central-Type Early Squamous Cell Lung Cancer
Planning in a Representative Case
A 66-year-old male patient with central early lung cancer (squamous cell carcinoma) located from the left main bronchus to the upper lobe bronchus was treated at our hospital. Irradiation with carbon ion beams at 61.2 GyE in 9 fractions within 3 weeks was performed in November of 2001. The treatment planning is shown in Fig. 21.4. Eight years after treatment, he is still alive and well without recurrence. No severe reaction was found on the follow-up CT or bronchoscopic examination.
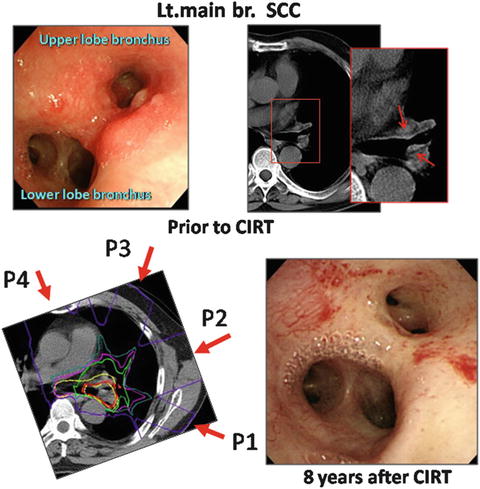

Fig. 21.4
Endoscopic findings prior to CIRT (left upper panel) and CT images. The tumor is shown by arrows. The planning CT images, where P1:P2:P3:P4 = 2:2:3:2, and P3 is a small target (left lower panel). An endoscopic image 8 years after CIRT (right lower panel)
21.3.2.3 Early-Stage NSCLC with an Extrabronchial Tumor Near the Hilus
Planning in a Representative Case
A 64-year-old male patient with left upper lobe (S4) lung cancer (squamous cell carcinoma) that measured 34 × 24 mm who also had hilar lymph node metastases was diagnosed with cT2N1M0 disease. Carbon ion radiation at 68.4 GyE was performed. As of 4 years after treatment, he is still alive and well, without any recurrence (Fig. 21.5).
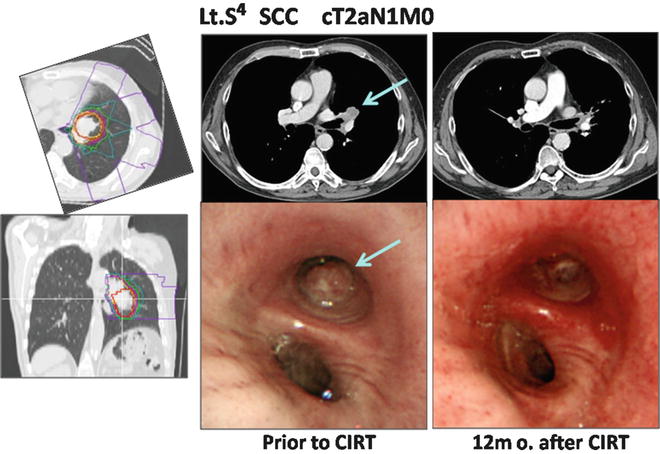

Fig. 21.5
The planning CT showing the four-portal irradiation. CT and endoscopic images taken pre- and post-therapy are shown. The arrow indicates the tumor
21.4 Locally Advanced Lung Cancer
21.4.1 Treatment Planning
Radiotherapy for advanced lung cancer is generally performed in combination with chemotherapy or surgery. During the CIRT for lung cancer, we treated some locally advanced lung cancer cases with CIRT alone. Because, these patients were unable to receive chemotherapy, or the cases of progressive disease after chemotherapy.
We have been making an extensive effort to minimize the risk of pulmonary, tracheal, and esophageal toxicity associated with the treatment. Using a small CTV for the primary lesion allows us to decrease the prescribed dose for the mediastinum. This will help improve the patients’ overall status, potentially increasing the number of patients who can receive chemotherapy.
21.4.2 Planning in Representative Cases
Case 1
A 75-year-old male patient with right lower lobe (S6) lung cancer (adenocarcinoma) measuring 40 × 38 mm, with chest wall infiltration and nodal metastasis of the right hilum, was diagnosed to have cT3N1M0 stage IIIA disease. Carbon ion beam irradiation at 72.0 GyE in 16 fractions over 4 weeks was performed in January 2004. Figure 21.6 shows a CIRT planning image.
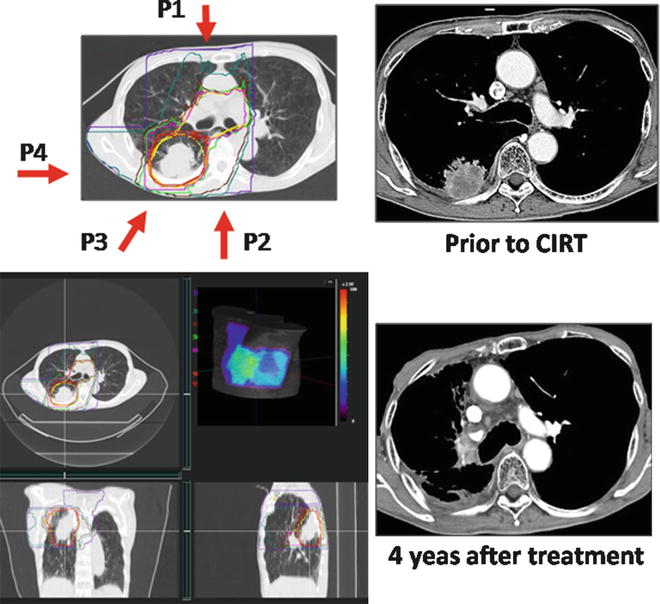
Fig. 21.6
The left panel shows the CTV and planning dose distribution, including 3D images and the body surface model. The irradiation times of each port were P1:P2:P3:P4 = 4:3:3:6. P4 is the small target for the primary tumor. The right panel shows the pre- and post-CIRT images
The entire large target, including the primary tumor, hilum, and mediastinum, was irradiated at 45.0 GyE, and then the primary tumor was irradiated to a final dose of 72.0 GyE. At 4 years after treatment, he was well without any recurrence.
Case 2
A 67-year-old male patient with left upper lobe lung cancer (adenocarcinoma) of 62 × 50 mm, with infiltration into the mediastinum, was diagnosed with cT4N0M0 stage IIIB disease. Irradiation with carbon ions at 72.0 GyE was performed. At 10 years after treatment, he is still alive and well without any recurrence (Fig. 21.7).
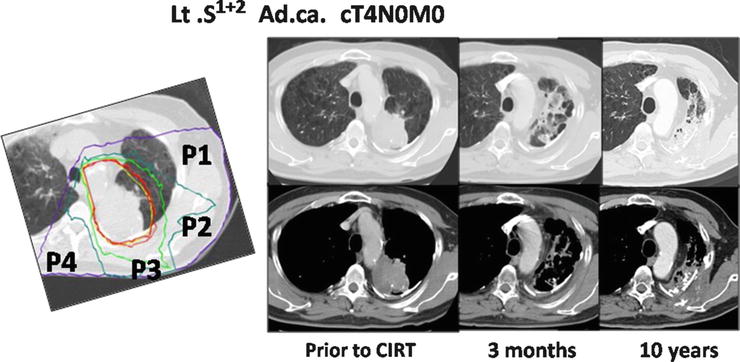
Fig. 21.7
The planning CT showing the four-portal irradiation. The irradiation times for each port were P1:P2:P3:P4 = 4:4:5:3. The CT images taken pretreatment and 3 months and 10 years after therapy are shown
21.4.3 Results
Sixty-one patients were treated in this clinical trial between May 2000 and September 2011. The 61 primary tumors were treated by carbon ion beam irradiation alone using a total dose of 72.0 GyE in 16 fractions over 4 weeks. The mean age of the patients was 74.2 years (46–88), and the gender breakdown was 14 females and 47 males. By histological type (the cancer type was determined by biopsy), there were 27 adenocarcinomas, 31 squamous cell carcinomas, and three large cell carcinomas. Of the 61 patients, 39 were in stage II and 22 were in stage III, while there were 24 N1 cases, 13 N2 cases, and 24 N0 (T3-4N0M0) cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree