Lung Cancer
Todd M. Blodgett, MD
Carl Fuhrman, MD
Sanjay Paidisetty, BS
Key Facts
Terminology
Non-small cell lung cancer (NSCLC), bronchogenic carcinoma (CA), adenocarcinoma (adenocarcinoma), squamous cell carcinoma (SCCA), large cell carcinoma (LCCA), bronchoalveolar carcinoma (BAC)
Imaging Findings
Spiculated pulmonary nodule in a smoker
Hilar and mediastinal lymphadenopathy
Whole body PET/CT is superior to CT or PET alone
PET/CT currently best technique for imaging diagnosis of nodal staging in NSCLC
PET can identify metastases that would have been occult on conventional workup (e.g., adrenal metastases)
Prognostic information from FDG PET: Max SUV > 5.0 suggests aggressive neoplasm, poorer prognosis
Top Differential Diagnoses
Pulmonary Infarct, Infection
Granulomatous Disease
Mediastinal Mass
Hamartoma
Infection
Pathology
> 85% smoke; 50% in former smokers
Diagnostic Checklist
Consider PET/CT for all patients with newly diagnosed NSCLC
Suspicious FDG PET findings should be biopsied/removed without following growth on CT
Suspicious CT morphology should be biopsied even with negative PET
TERMINOLOGY
Abbreviations and Synonyms
Non-small cell lung cancer (NSCLC)
Adenocarcinoma
Bronchogenic carcinoma
Squamous cell carcinoma (SCCA)
Bronchoalveolar carcinoma (BAC)
Large cell carcinoma (LCCA)
Definitions
Glandular carcinomas of varying histology arising in lung parenchyma
IMAGING FINDINGS
General Features
Best diagnostic clue
Peripheral irregular spiculated pulmonary nodule in a smoker
Malignant in > 90% of patients
Hilar and mediastinal lymphadenopathy
Bronchial stenosis and associated atelectasis
Location
Adenocarcinoma: Periphery of upper lobe
SCCA: Central
Size
By time of detection
At screening: 8-15 mm
Symptomatic: 25 mm
Size is not a reliable indicator of nodal involvement
21% of subcentimeter nodes = malignant
40% of nodes > 1 cm = benign
LCCA: > 4 cm at diagnosis
Morphology
Spiculated irregular ill-defined nodule is specific for malignancy
Borders may also be lobulated or smooth
Imaging Recommendations
Best imaging tool
CT for delineating extent of disease
PET/CT for prognosis and staging
MR for diagnosing neural invasion
CNS, Pancoast invasion of brachial plexus
No imaging tool is specific enough to defer lymph node biopsy if required for treatment planning
Mediastinoscopy most common method for assessing mediastinal lymph nodes
Protocol advice
CT
To include adrenals, image caudally from thoracic inlet to inferior edge of liver
Pleural and diaphragmatic tumor spread best imaged with multiplanar reformatting
Screening CT studies should be performed without contrast and with the lowest possible mAs
FDG PET
Serum glucose < 150 mg/dL (reschedule hyperglycemic patients)
90 minute uptake period
PET/CT
Modified breath-hold techniques employed to reduce misregistration artifact
Sensitivity in lower lobes may be minimized without maximal inspiration
CT Findings
General findings
Current CT does not provide adequate anatomic detail to separate invasion from simple contact between tumor and adjacent organ
NECT adequate for evaluation of primary tumor, but less sensitive for vascular invasion and liver metastases
CECT can reveal small endobronchial lesions and better elicit small mediastinal nodes
Particularly hilar nodes that may otherwise missed due to proximity to vessels
Bronchial obstruction with lobar collapse or post-obstructive pneumonitis
Peripheral lesions
SCCA more likely to demonstrate cavitation
Peripheral adenocarcinoma may be solid, mixed solid/ground-glass, or ground-glass
Staging
CT has not demonstrated improved staging accuracy with the advent of multislice detectors
Malignant potential and degree of contrast enhancement may be related due to increased vascularity of lung cancer lesions
Lymph nodes
Upper size limits for suspicion of malignancy
> 5 mm hilum and > 10 mm mediastinum
Size criteria are insensitive, as normal-sized nodes often harbor malignancy
Sensitivity 41-54%
Combined PET/CT may correct 81% of false negatives on CT
Measurement errors inherent with borderline node size of 5 mm
Mediastinal and hilar nodal metastasis is not predicted by characteristics of primary tumor
Size, margins, necrosis, bronchovascular thickening
Best predictor of mediastinal nodal metastasis is peak enhancement of malignant lung nodules
> 100 HU or > 60 HU of net enhancement in stage T1 NSCLC
Mediastinoscopic biopsy recommended in the setting of these findings whether FDG PET is positive or not
Supraclavicular nodal sensitivity of 67-85%
Area often obscured by beam-hardening artifacts
Delayed scans may improve detection
Transverse scan may not accurately depict short-axis measurement of nodes
Normal structures that mimic lymph nodes
External/internal jugular veins
Vertebral veins
Common carotids
Scalene and longus colli muscles
Metastatic disease
High resolution CT to detect lymphangitic carcinomatosis
Look for metastasis to non-tumor lobe
CECT can demonstrate pleural metastases in pleural effusion
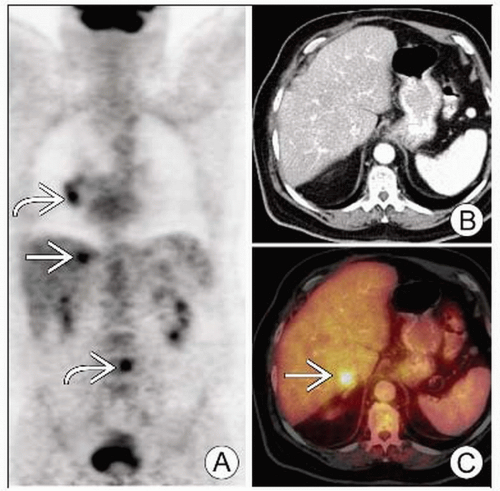
Adrenal evaluation complicated by insensitivity of morphologic criteria for presence of malignancy
May be evaluated with NECT
17% of normal-appearing adrenals may harbor malignancy
Overall, fewer than half of adrenal masses in patients with lung cancer will be due to metastasis
Nuclear Medicine Findings
PET/CT
Central to initial and post-treatment evaluation and management of NSCLC
Depicts response to treatment accurately and predicts prognosis
Influences management by demonstrating occult disease
Provides high contrast between tumor and adjacent structures like mediastinum, chest wall, atelectasis
Helps determine cause of pleural effusion
Normal uptake
Low level in thyroid, breast, and mediastinal blood pool
Talking can cause laryngeal uptake
Anxiety may be manifested as uptake in the SCM
Brown fat in neck, paravertebral, mediastinal, and axillary regions
Also seen in esophagus, spleen, liver, and bowel
Initial diagnosis
FDG PET criteria for malignancy (less specific than sensitive)
FDG uptake > background mediastinal uptake
Max SUV ≥ 2.5; however wide overlap of SUVs between benign and malignant processes
Any activity above background levels in lesion < 1-1.5 cm
For SPN larger than 1.0 cm, PET has sensitivity and specificity of 95-98% and 73-85% respectively
Same predictive value as observation of nodule growth on CT
Nodules < 2.5 cm may be misinterpreted as non-FDG-avid due to partial volume averaging on PET
Recovery coefficient helps correct SUV in smaller nodules for purposes of quantitative determinations
Tumor with low metabolic rate (low grade adenocarcinoma, BAC, carcinoid)
Subtypes may show low FDG uptake despite aggressiveness
BAC, carcinoid, low grade adenocarcinoma
Staging
Detection of disease
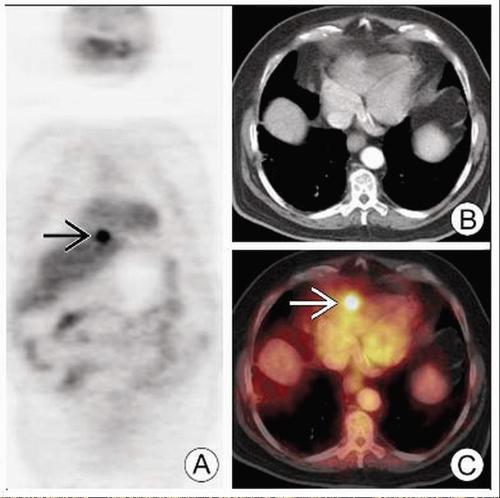
PET can identify metastases that would be occult on conventional workup (e.g., adrenal metastases)
Detects bone metastases with equal sensitivity to and greater specificity than bone scintigraphy
False positives with benign inflammatory disease > 1.0 cm
False negatives in subcentimeter lesion with limited cancer invasion
Low sensitivity of 47% for stage T1 NSCLC
100% PPV and high NPV for mediastinal nodal metastasis
Brain metastases may be overlooked due to high background FDG avidity
Necrotic metastases may be negative (e.g., adrenal masses)
Influence on management
PET more sensitive than clinical exam for supraclavicular lymphadenopathy
Significant indicator of inoperable disease
Confirmation by histopathology is mandatory when positive PET/CT would deny patient chance for potentially curative treatment
High NPV of 93% helps avoid invasive procedures like fine needle aspiration (FNA) when there is no FDG uptake
Microscopic disease may be present, and mediastinal surgical staging may still be indicated
Thoracotomy
PET has greater than 90% NPV for nodal disease
Patients with negative mediastinal nodes may proceed directly to thoracotomy without need for mediastinoscopy
PET may also avoid non-therapeutic thoracotomy in 20% by detecting previously occult distant disease
PET/CT may alter 50% of radiotherapy gross tumor volume estimation compared to targeting with CT alone
Clinical impact yet to be determined
Gross tumor volume (GTV) shown to include all pathologically involved mediastinal lymph nodes
More positive lymph nodes are detected by PET/CT compared to CT alone
Prognosis
FDG PET may provide information on prognosis independent from standard staging algorithms
Primary tumor with SUV ≥ 5.0
Associated with significant increase in post-operative relapse in early stage lung cancer
Intense uptake in bone marrow also associated with poorer outcome
Response to treatment
Decrease in FDG avidity of malignant lesion by 60% following 2-3 cycles of chemotherapy
May indicate good response and be predictive of improved survival
Some tumors may show falsely decreased SUV post-therapy (“stunned”) but still represent threat of recurrence
False positives
Nonmalignant metabolically active conditions
Active inflammation, infection, granulomatous disease
Pattern of physiologic muscle uptake typically
Bilateral, symmetric, fusiform, or elongated; seldom confused with presence of malignancy
Asymmetric uptake can occur
Increased glycolysis in leukocytes, lymphocytes, macrophages
Produce uptake in areas of infection, inflammation
Examples of benign processes that may mimic malignancy
Atherosclerotic plaque
Reflux esophagitis
Tuberculous caseating granuloma
Sarcoidosis
Wegener granulomatosis
Amyloidosis
Pulmonary infarction
Pulmonary embolus
Pulmonary hamartomas
Needle biopsy site
Mediastinoscopy
Talc pleurodesis, which may remain FDG positive for several years
Empyema
Pneumonias usually produce low grade uptake but avid uptake can occur
Organizing pneumonia as a single focus of consolidation can mimic lung cancer
Patients with false positives on PET/CT often have comorbid pulmonary complications, such as
Obstructive pneumonia
Chronic bronchitis
Interstitial pneumonia
Bronchiectasis
Silicosis
Previous pulmonary tuberculosis
DIFFERENTIAL DIAGNOSIS
Metastases
Usually less spiculated than primary pulmonary malignancies
Variably FDG avid
Infection
Pneumonia may show intense focal uptake and is easily mistaken for malignancy
Repeat PET to rule out presence of underlying malignancy
Granulomatous Disease
Mediastinal and hilar adenopathy generally symmetrical and FDG avid
Predominantly in upper lobes
Calcification is common
Pulmonary Infarct
Early following infarct, may show intense FDG activity
Usually becomes less intense over time
Distribution of occluded artery
Hamartoma
Generally noninflammatory with little FDG uptake
“Popcorn” calcifications
PATHOLOGY
General Features
General path comments: Sputum analysis is insensitive, with false negative in 40% of cases
Genetics
NSCLC associated with amplification of oncogenes (e.g., ras family) in 30% of cases
Also inactivation of tumor suppression genes
Portends worse prognosis
Genetic predisposition plays role in vulnerability to risk factors (e.g., smoking)
Etiology
Tobacco is the cause of lung cancer in ˜ 90% of cases
Disease was practically unknown prior to the rise of cigarette smoking in the 1920s
15% of lung cancer in patients who do not smoke is caused by passive smoke
Asbestos exposure in a patient who smokes confers 80-90x increased risk of lung cancer
Radon accounts for 2-3% of lung cancer and is a byproduct of uranium decay (miners)
Epidemiology
In USA: 170,000 new cases/year (90k in men and 80k in women); 150,000 deaths/year
Estimated 1 million new cases/year worldwide
NSCLC accounts for ˜ 80% of all lung cancers
Associated abnormalities: COPD
Gross Pathologic & Surgical Features
Cavitation is common in SCCA
Microscopic Features
Adenocarcinoma: Forms glands and produces mucin, which can be identified with mucicarmine or PAS staining
SCCA: Large irregular nuclei, coarse nuclear chromatin, large nucleoli
Presence of intercellular bridging among cells arranged in sheets is pathognomonic
LCCA: Large cells with prominent nucleoli in the absence of mucin production or intercellular bridging
Staging, Grading, or Classification Criteria
Primary lesion (T)
Tx: Tumor cannot be assessed, + sputum/washings
T0: No evidence of primary tumor
Tis: Carcinoma in situ
T1: < 3 cm, completely surrounded by lung; no main bronchi invasion
T2: > 3 cm, involving main bronchus > 2 cm from the carina, visceral pleura; atelectasis/pneumonitis extending to hila
T3: Invading chest wall, diaphragm, mediastinal pleura, parietal pericardium, main bronchus < 2 cm from the carina
Total atelectasis of whole lung
T4: Invading the mediastinal structures, vertebrae, carina; separate tumor nodules in same lobe; malignant pleural effusion
Mediastinal and hilar lymph nodes (N)
N1: Hilar lymph nodes at vessel branch point
N2: Ipsilateral to primary tumor (subcarinal lymph node is ipsilateral)
N3: Contralateral lymph node, supraclavicular fossa
Stages
CLINICAL ISSUES
Presentation
Most common signs/symptoms
Central primary tumor
Cough, dyspnea, wheezing, and hemoptysis
Atelectasis and post-obstructive pneumonia
Peripheral primary tumor may cause the above
± Pleural effusion and pain related to pleural and chest wall invasion
Locoregional spread may cause SVC obstruction and nerve infiltration
Leads to hoarseness, diaphragm paralysis, and Horner syndrome, as well as brachial plexus neuropathy
Paraneoplastic symptoms may include hypercalcemia and PTHrP production
Other signs/symptoms
Recurrent pneumonia in same lobe
Metastasis to supraclavicular lymph node
Indicator of inoperable disease
Palpation of supraclavicular lymph nodes is unreliable for detection
Demographics
Age: > 50 years
Gender
M:F approaching 1:1 in USA
M > F worldwide
Mortality higher in males, increasing in females
Ethnicity: African-American:Caucasian:Native American = 1.5:1:0.2
Natural History & Prognosis
Most patients present with advanced disease
Adenocarcinoma has worse prognosis per stage than SCCA (except T1N0)
Staging criteria include
Histologic type
Tumor size
Regional lymph node involvement
Presence of metastatic disease
Mediastinal lymph node metastasis is found in 16-21% of patients with stage T1 NSCLC
Metastatic relapse occurs in up to 20% of patients who have undergone surgery
Treatment
Stages 1 & 2: Patients with no metastatic lymph nodes (N0 disease) or with only intrapulmonary or hilar lymph nodes (N1 disease)
Resection with adjuvant chemo in selected cases
Stage 3A: Neoadjuvant chemoradiation, then surgery in selected
Stage 3B: Chemoradiation, then surgery in selected T4N0
Stage 4: Chemotherapy, palliative radiation in selected
Solitary brain mets: Resection of brain met and primary if possible
Radiation or RFA: Symptomatic inoperable lesions
Some patients with inoperable NSCLC can be cured with radiotherapy
DIAGNOSTIC CHECKLIST
Consider
Suspicious CT morphology should be biopsied even with negative PET
Suspicious FDG PET findings should be biopsied/removed without following growth on CT
Consider PET/CT for ALL patients with newly diagnosed NSCLC
SELECTED REFERENCES
1. Bryant AS et al: Differences in outcomes between younger and older patients with non-small cell lung cancer. Ann Thorac Surg. 85(5):1735-9; discussion 1739, 2008
2. Cerfolio RJ et al: Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann Thorac Surg. 86(2):362-6; discussion 366-7, 2008
3. Decker RH et al: Advances in radiotherapy for lung cancer. Semin Respir Crit Care Med. 29(3):285-90, 2008
4. Hampton T: New studies target lung cancer prevention, imaging, and treatment. JAMA. 300(3):267-8, 2008
5. Hanin FX et al: Prognostic value of FDG uptake in early stage non-small cell lung cancer. Eur J Cardiothorac Surg. 33(5):819-23, 2008
6. Hart JP et al: Radiation pneumonitis: correlation of toxicity with pulmonary metabolic radiation response. Int J Radiat Oncol Biol Phys. 71(4):967-71, 2008
7. Higaki F et al: Preliminary retrospective investigation of FDG-PET/CT timing in follow-up of ablated lung tumor. Ann Nucl Med. 22(3):157-63, 2008
8. Kased N et al: Prognostic value of posttreatment [18F] fluorodeoxyglucose uptake of primary non-small cell lung carcinoma treated with radiation therapy with or without chemotherapy: a brief review. J Thorac Oncol. 3(5):534-8, 2008
9. Lima CG Jr et al: Is there a definitive answer to the question of involved-field radiotherapy for inoperable non-small-cell lung cancer? J Clin Oncol. 26(13):2235; author reply 2235-6, 2008
10. Ohno Y et al: Non-small cell lung cancer: whole-body MR examination for M-stage assessment—utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology. 248(2):643-54, 2008
11. Yi CA et al: Non-small cell lung cancer staging: efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology. 248(2):632-42, 2008
12. Poettgen C et al: Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 73(5-6):316-23, 2007
13. Decoster L et al: Complete metabolic tumour response, assessed by 18-fluorodeoxyglucose positron emission tomography ((18)FDG-PET), after induction chemotherapy predicts a favourable outcome in patients with locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. (In Press)
14. Wilson DO et al: The Pittsburgh Lung Screening Study (PLuSS): Outcomes within 3 years of a first CT Scan. Am J Respir Crit Care Med. (In Press)