Etiology
Lung cancer arises when cells lining the bronchi or peripheral airways undergo neoplastic change in response to an inciting agent or agents. The most common and well-recognized cause of lung cancer is cigarette smoking, which has been unequivocally linked with this disease through case-control studies since the 1950s. A dose-response relationship between cigarette consumption and the development of lung cancer has been well established. Although all forms of lung cancer are associated with cigarette smoking, squamous cell and small cell carcinomas of lung are more closely related to the intensity of smoking than is adenocarcinoma. In addition to the association between active smoking and lung cancer, second-hand smoke exposure is associated with a 20% to 30% increase in the risk of lung cancer among nonsmoking adults.
Although approximately 85% of all cases of lung cancer in North America and Europe can be attributed to cigarette smoking, many additional risk factors have been identified, most of which are environmental or occupational in nature. The most well-recognized occupational exposure associated with lung cancer is asbestos, which itself produces a 7- to 10-fold increase in the risk of lung cancer and has a synergistic effect with cigarette smoke on lung cancer development. Other established exposures associated with an increased risk include silica, radiation (i.e., radon, x-rays, and gamma rays), air pollution, arsenic, cadmium, and chromium. Intrinsic risk factors for the development of lung cancer include the presence of chronic obstructive pulmonary disease (COPD), emphysema, and interstitial fibrosis. In recent years lung cancer susceptibility risk foci have been mapped to various chromosomal regions, such as 15q and 5p, in different ethnic populations in genome-wide association studies.
Prevalence and Epidemiology
Lung cancer is the most common non–skin cancer worldwide both in terms of incidence and mortality. It is estimated that globally, in 2012, 1.8 million new cases of lung cancer were diagnosed (12.9% of total cancer incidence), and 1.59 million lung cancer deaths occurred (19.4% of all cancer deaths). Lung cancer is responsible for more deaths in the United States than the next three most commonly diagnosed cancers (colon, breast, and prostate) combined. Adenocarcinoma has become the most common subtype of lung cancer both in smokers and nonsmokers.
The incidence and death rates from lung cancer in the United States have declined with increased awareness of hazards associated with smoking and smoking restrictions in public areas. The incidence of lung cancer increases with increasing age. The median age at time of lung cancer diagnosis during the 2008 to 2012 period was 70 years, with a median age at death of 72 years.
There are specific racial, ethnic, geographic, and socioeconomic associations with lung cancer incidence. Central and eastern Europe and eastern Asia have the highest incidence rates of lung cancer in men (50–54 per 100,000), whereas North America and northern Europe have the highest estimated incidence for women (17–34 per 100,000). In the United States, African Americans have higher incidence and mortality rates (68 and 48 per 100,000 in 2012, respectively) compared with Caucasians (54 and 46 per 100,000, respectively). The 5-year survival rate in the United States for 2011 was 18.0%.
Clinical Presentation
Asymptomatic
It is estimated that 25% of patients with lung cancer are asymptomatic at the time of diagnosis. It is likely that as the indications for the use of computed tomography (CT) in the evaluation of pulmonary and cardiovascular diseases expand, the incidental detection of asymptomatic lung cancers will increase. In particular, with increasing use of low-dose CT to screen for asymptomatic lung cancer, it is likely that more lung cancers will be detected at an asymptomatic point in the natural course of the disease.
Symptomatic
Because many patients with lung cancer have concomitant chronic obstructive pulmonary disease (COPD), symptoms caused by a developing lung cancer may mimic an exacerbation of COPD or be masked by the chronic cough and shortness of breath commonly associated with this condition. The main symptoms associated with lung cancer depend on the site of origin of the primary tumor and the pattern of spread within the thorax and body. Although there are likely numerous genetic, host immune, and anatomic factors that affect the natural history of lung cancer and determine the presenting symptoms and signs of disease, the clinical presentation of lung cancer is most closely associated with the specific cell type of lung cancer as seen on light microscopy.
Squamous cell carcinoma and small cell carcinoma most often arise from a central (i.e., main, lobar, or segmental) bronchus with early spread to regional hilar and mediastinal lymph nodes. As a result, these tumors most often produce symptoms associated with central airway involvement, including cough, hemoptysis, shortness of breath, wheezing, and post–obstructive pneumonia or abscess formation. In addition to airway compromise, large central or paramediastinal tumors and lymph node masses can result in symptoms related to local compression or invasion of mediastinal structures, such as hoarseness from medial left upper lobe tumors or aorticopulmonary window lymph node masses invading the left recurrent laryngeal nerve, diaphragmatic dysfunction from phrenic nerve involvement, or dysphagia as a result of esophageal compression or invasion. Locally invasive superior sulcus (Pancoast) tumors can produce Horner syndrome (triad of miosis [constricted pupil], partial ptosis, and hemifacial anhidrosis [loss of sweating]) from sympathetic chain involvement, shoulder pain, and brachial plexopathy. Large medial right upper lobe and paratracheal lymph node masses can produce superior vena cava syndrome. Paracardiac tumors can extend into the heart, most often via the pulmonary vein, or into the pericardium, leading to systemic tumor embolization or pericardial effusion. Peripherally situated tumors can produce chest pain as a result of parietal pleural and chest wall invasion, often with associated pleural effusion. Shortness of breath can result from post–obstructive pneumonitis or atelectasis, lymphangitic spread or tumor in the affected lung or lobe, or malignant pleural effusion. Rarely, a cavitary peripheral tumor, such as a squamous cell carcinoma, can rupture through the visceral pleura and manifest with a spontaneous pneumothorax.
Because approximately 40% to 50% of patients with non–small cell lung cancer and most patients with small cell carcinoma of the lung have metastatic disease at presentation, their clinical symptoms are often manifestations of extrathoracic involvement. Because the most common sites of metastatic lung cancer include the bones, liver, brain, lung, and adrenal glands, common presenting symptoms include headache, seizures, bone pain (often from pathologic fracture), anorexia, weight loss, and rarely adrenal insufficiency.
Of patients with lung cancer, 10% to 15% present with paraneoplastic syndromes. Most paraneoplastic syndromes are associated with small cell carcinoma, with neuromuscular and endocrine syndromes, including Lambert-Eaton syndrome (i.e., muscle weakness that improves with continued contraction of the muscle), syndrome of inappropriate antidiuretic hormone, and Cushing syndrome, the most common. Humoral hypercalcemia of malignancy secondary to lung cancer is most often associated with squamous cell carcinoma and is the result of a parathyroid hormone–related protein secreted by the tumor.
Pathology
The 2015 World Health Organization (WHO) classification system for lung tumors incorporates recent advances in clinical, radiologic, histologic, and genetic aspects of lung cancer. Exact histopathologic subtyping of lung cancer has become increasingly important in the past decade with the discovery of new therapeutic options that specifically target certain types of lung cancers.
Adenocarcinoma is the most common histologic subtype of lung cancer. Microscopically, adenocarcinoma cells are large and cuboidal, columnar, or polygonal in shape with prominent nucleoli ( Fig. 17.1 ). These malignant tumors usually arise from the cells lining the distal bronchioles and alveoli and are seen grossly as well-circumscribed lobulated solid nodules surrounded by lung, with tumors that vary in size from less than 10 mm in diameter to the size of the entire lung. Scarring often associated with adenocarcinoma produces puckering of adjacent pleural surfaces. Classification of adenocarcinoma has changed significantly based on the 2015 WHO classification ( Box 17.1 ). Invasive adenocarcinomas are further classified based on the predominant growth pattern, such as lepidic, acinar, papillary, solid, or micropapillary. The term bronchioloalveolar cell carcinoma is no longer used. Instead, lepidic pattern is used to describe neoplastic cell growth along the alveolar septa with no architectural disruption and no evidence of lymphovascular or pleural invasion. Tumors that show a predominant lepidic (i.e., bronchioloalveolar) pattern but do contain invasive components are classified as lepidic adenocarcinoma. The terms adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) have been introduced to describe small lesions with a pure and predominant lepidic pattern that have a 100% cure rate with resection. The pathologic criteria for AIS and MIA are listed, respectively, in Boxes 17.2 and 17.3 . Atypical adenomatous hyperplasia (AAH) is considered a precursor or preinvasive lesion in the adenocarcinoma spectrum. Immunocytochemical analysis of lung adenocarcinoma shows positive staining for the presence of thyroid transcription factor-1, a feature that helps to distinguish primary lung adenocarcinoma from metastatic adenocarcinoma of the breast or gastrointestinal tract. Because of the availability of new effective, targeted therapeutic agents, molecular testing for epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangements of lung adenocarcinoma is now recommended by various professional societies.

- •
Lepidic
- •
Acinar
- •
Papillary
- •
Micropapillary
- •
Solid
- •
Invasive mucinous
- •
Colloid
- •
Fetal
- •
Enteric
- •
Minimal invasive
- •
Nonmucinous
- •
Mucinous
- •
- •
Preinvasive lesions
- •
Atypical adenomatous hyperplasia
- •
Adenocarcinoma in situ
- •
Nonmucinous
- •
Mucinous
- •
- •
- •
Solitary tumor ≤3 cm
- •
Pure lepidic growth
- •
No stromal, vascular, or pleural invasion
- •
No pattern of invasive adenocarcinoma (such as acinar, papillary)
- •
No spread through airspaces
- •
Cell type mostly nonmucinous
- •
Nuclear atypia absent or inconspicuous
- •
Solitary tumor ≤3 cm
- •
Predominantly lepidic growth
- •
≤0.5-cm invasive component in greatest dimension in any one focus
- •
Invasive component includes any histologic subtype other than a lepidic pattern (such as acinar, papillary, etc.)
- •
Tumor cells infiltrating myofibroblastic stroma
- •
Cell type mostly nonmucinous
- •
MIA diagnosis excluded if the tumor contains necrosis, spreads through airspaces or if the tumor invades lymphatics, blood vessels, airspaces, or pleura
Squamous cell carcinoma (SCC) is recognized pathologically by the presence of nests of malignant cells with associated keratinization and intercellular bridges ( Fig. 17.2 ). In the absence of clear evidence of keratinization, immunohistochemistry with positive squamous markers, such as p40 or p63, is required to diagnose nonkeratinizing SCC. Based on the updated WHO classification, SCC now consists of keratinizing, nonkeratinizing, and basaloid subtypes. Most of these tumors arise within the segmental or subsegmental bronchi and are generally smaller than other lung cancers at the time of presentation because they produce masses in the central regions of the lung that produce symptoms of obstruction or cough. Macroscopically, these tumors manifest as solid masses, commonly associated with necrosis and hemorrhage; cavitation is seen in one-third of lesions.

Small cell lung carcinoma (SCLC) is the most common form of neuroendocrine tumors of the lung and accounts for approximately 15% to 20% of all lung cancers. Other neuroendocrine tumors include carcinoid tumor (discussed in detail in Chapter 19 ) and large cell neuroendocrine carcinoma (LCNEC). SCLC has distinct microscopic features that include small size cells with a round shape, large nuclei with granular (i.e., salt-and-pepper) appearance, and scant cytoplasm ( Fig. 17.3 ). Regions of necrosis are common, and mitoses are frequent (>10 mitoses per 2 mm 2 ). These tumors usually arise centrally, infiltrate the peribronchial tissues, and often are associated with regional lymph node metastases at the time of diagnosis. Immunohistochemistry markers for neuroendocrine tumors include chromogranin, synaptophysin, and CD56. Ki-67 is used to separate the high-grade SCLC and LCNEC from low- and intermediate-grade carcinoid tumors.

LCNEC comprises approximately 3% of lung cancers and clinically behaves in an aggressive manner, with poor prognosis similar to SCLC. The cytologic features of large cell size and low nuclear to cytoplasmic ratio are similar to non–small cell lung carcinoma; however, LCNEC has high mitotic rate and demonstrates positivity for one or more neuroendocrine markers on immunohistochemical staining.
Lung Function
Pulmonary functional abnormalities are common in patients with lung cancer but are nonspecific because they generally reflect the presence of underlying lung disease. Chronic hypoxemia with compensated respiratory acidosis and expiratory airflow limitation in lung cancer patients are common manifestations of underlying COPD. An acute deterioration in blood oxygen saturation levels should suggest the development of obstructive pneumonitis, a malignant pleural effusion, or complicating pulmonary embolism. Less commonly, restrictive lung disease is seen, particularly in patients with lung cancer complicating idiopathic pulmonary fibrosis. The development of diminished diffusing capacity and decreased lung volumes in a patient with lung cancer in concert with developing interstitial changes on chest radiography or CT should suggest the possibility of lymphangitic carcinomatosis. Rarely, the presence of a centrally obstructing tracheal or main bronchial lesion is suggested by characteristic changes on a flow/volume loop.
Patients with potentially resectable lung cancer should routinely undergo pulmonary function testing, including measurement of forced expiratory volume in 1 second (FEV1) obtained by spirometry and diffusing capacity for carbon monoxide (DLCO). The type and volume of lung to be resected factors into the decision of whether resection can be performed safely in patients with lung functional abnormalities, with more strict functional exclusionary criteria for patients requiring pneumonectomy compared with lobectomy, segmentectomy, or localized wedge resection. Patients being considered for lobectomy who have an FEV1 of less than 1.5 L may require cardiopulmonary exercise testing with measurement of maximal oxygen consumption and quantitative ventilation/perfusion scanning to assess more accurately for perioperative risk of complications before potential curative lung resection. An FEV1 or DLCO less than 40% of predicted and a maximal oxygen consumption less than 15 mL/kg/min are associated with an increased risk of perioperative complications.
Manifestations of the Disease
A familiarity with the spectrum of imaging manifestations of lung cancer should facilitate the timely and accurate diagnosis of the disease, directing the appropriate imaging, surgical, and oncologic evaluation to stage the disease and determine the appropriate management.
Radiography
Solitary Pulmonary Nodule or Mass
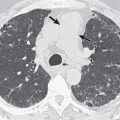
Important radiographic features in the evaluation of a solitary pulmonary nodule (SPN) or mass include change in size over time, margins, and the presence of calcification (see Chapter 4 ). The first step in the evaluation of a radiographically detected SPN is to determine if the nodule was present on prior radiographs. The confirmation of a stable SPN 2 years or longer of duration is strong evidence of benign status. In general, any growth in an SPN or an SPN that was not evident on prior radiographic examinations requires CT for further evaluation. Irregularly marginated lesions, particularly lesions with spiculated borders ( Fig. 17.4 ), are viewed with concern and almost invariably warrant further evaluation, whereas a smooth margin generally indicates a benign lesion. The presence of diffuse, central, laminar, or popcorn calcification signals a benign process, such as granuloma or hamartoma, whereas other types of calcification require further evaluation. Occasionally, the radiographic detection of feeding or draining vessels or a comet tail entering the hilar aspect of a subpleural round lesion is evidence of a benign lesion, specifically an arteriovenous malformation and rounded atelectasis, respectively.

Lobar or Segmental Consolidation or Atelectasis
The detection of lobar opacification with or without volume loss in an adult patient should always be viewed with concern because pneumonia involving an entire lobe is uncommon in the modern antibiotic era, and lung cancer may manifest in this manner ( Fig. 17.5 ). The mechanism of lobar opacification is usually due to post–obstructive atelectasis ( Fig. 17.6 ) or endogenous lipoid pneumonia distal to a centrally obstructing mass, most typically squamous cell or small cell carcinoma. Rarely, mucinous adenocarcinoma may manifest as segmental or lobar consolidation ( Fig. 17.7 ), often associated with ill-defined nodular opacities. In patients with lobar or segmental opacity, the absence of air bronchograms; a central convexity to the opacified lobe (termed the S sign of Golden to signify the contour of the obstructed, atelectatic lobe along with contiguous centrally obstructing mass or regional lymph node involvement, as outlined by adjacent aerated lung); lack of improvement for treated asthma, bronchitis, or pneumonia; and associated hemoptysis or weight loss are clues to the diagnosis and should prompt CT or bronchoscopic evaluation or both.



Hyperlucent Lung
Occasionally, a central tumor may result in partial obstruction of a main or lobar bronchus and hyperlucent lung distal to the mass. Patients who present with hyperlucent lung secondary to lung cancer often have associated cough, hemoptysis, or wheezing owing to the central location of the tumor. This finding is more commonly associated with slow-growing central tumors such as carcinoid tumors, which have intraluminal and extraluminal components.
Hilar or Mediastinal Mass
The most common cause of a unilateral hilar or middle mediastinal mass in adults is lung cancer, with the mass reflecting central tumor or regional lymph node enlargement (see Fig. 17.5 ). Squamous cell and small cell carcinoma are the most frequent cell types to produce a mediastinal or hilar mass, although approximately 25% of adenocarcinomas manifest in this manner. These masses are often associated with central airway compromise, producing segmental, lobar, or complete lung atelectasis, or vascular invasion, producing superior vena cava syndrome or lung infarction, the latter resulting from pulmonary arterial or venous invasion by the tumor. Uncommonly, a central lung cancer involves the phrenic nerve, resulting in diaphragmatic paralysis with elevation of the ipsilateral hemidiaphragm, or the recurrent laryngeal nerve, resulting in hoarseness.
Diffuse Interstitial Disease
Rarely, the initial chest radiographic findings in patients with lung cancer are primarily the findings of interstitial lung disease. Diffuse interstitial disease may be seen in patients who present with lung cancer associated with lymphangitic carcinomatosis, which appears as regional or diffuse linear opacities often with associated hilar and mediastinal lymph node enlargement, subpleural edema, and pleural effusions. A less common cause of interstitial disease in lung cancer patients is the presence of underlying chronic interstitial lung disease, most often idiopathic pulmonary fibrosis, which is associated with an increased incidence of lung cancer and can obscure the presence of the underlying malignancy.
Chest Wall and Lung Apex Involvement
Lung cancers that arise peripherally, particularly adenocarcinoma and Pancoast tumors arising in the lung apex ( Fig. 17.8 ), can occur as chest wall masses secondary to local chest wall invasion. Radiographically, these lesions may be difficult to distinguish from primary chest wall lesions, and biopsy is necessary for accurate diagnosis.