• The lymphomas are caused by the malignant clonal expansion of either T or B lymphocytes – these can accumulate within lymph nodes (causing lymphadenopathy) or infiltrate solid organs • NB: if malignant lymphocytic change predominantly involves the lymph nodes (± extranodal sites) this is described as a lymphoma • The defining cell is the Reed–Sternberg cell (usually derived from germinal centre B cells or, rarely, peripheral T cells) • These are more commonly seen with HD and include: fever • This is mainly a disease of the elderly, with a median age at diagnosis of 65 years (the incidence increases exponentially with age after 20 years) • HD is less common than NHL (3.7 vs 15.1/100 000/year, respectively) • Epstein–Barr virus (EBV): this is present in > 90% of cases of Burkitt’s lymphoma • Retrovirus human lymphotropic virus type 1 (HTLV-1): this has been implicated in the causation of adult T-cell lymphomas • Human herpes virus 8: implicated as a cause of primary effusion large cell lymphoma • Helicobacter pylori: this is necessary for developing a gastric lymphoma of the mucosa-associated lymphoid tissue (MALT) type • Localized disease (stages 1A and 11A): radiotherapy to the involved nodes as well as any adjacent nodes • Advanced disease (stages 11B, IIIA/B and IVA/B): extensive combination chemotherapy is used in the first instance (± subsequent consolidatory radiotherapy to any sites of ‘bulky’ disease to reduce risk of local recurrence) • Radiotherapy is avoided in young patients where possible • Unlike HD, the histological subtype is the major determinant of treatment • This is usually combination chemotherapy, since around 80% of patients will have advanced disease at presentation • A highly aggressive B-cell variant of NHL which is associated with the Epstein–Barr virus (EBV) • 2M:1F • Endemic (mainly African children) or non-endemic (mainly white children) • Extranodal disease is common and there is a risk of CNS disease (leptomeningeal disease can be seen at presentation and is a site of relapse) • Retroperitoneal and paraspinal disease can cause paraplegia (a presenting feature in up to 15%) • It accounts for only 2–3% of NHL in immunocompetent adults (but 30–50% of all childhood lymphomas) • Although these tumours are extremely aggressive, they are potentially curable (chemotherapy) Cotswold’s modification of the Ann Arbor staging classification of Hodgkin’s disease* • These arise from mucosal sites that normally have no organized lymphoid tissue, but within which acquired lymphoid tissue has arisen as a result of chronic inflammation or autoimmunity: • There is a median age of 60 years (F>M) • Sites: the GI tract is the commonest site (50%), and within the GI tract the stomach is the most often affected (85%) • There are four broad groupings associated with an increased incidence of lymphoma and lymphoproliferative disorders: • Lymphoma is the first AIDS-defining illness in up to 5% of HIV patients (the incidence of all subtypes of NHL is increased 60–200-fold and the incidence of HD is increased up to 8-fold) • Most tumours are aggressive, with advanced stage, bulky disease and a high serum LDH at presentation • Chest: NHL is usually extranodal • PCNSL: deep white matter lesions • This occurs in 2–4% of solid organ transplant recipient patients • Most are associated with EBV infection and appear to represent EBV-induced monoclonal or, more rarely, polyclonal B-cell or T-cell proliferation as a consequence of immune suppression • PTLD develops earlier in patients receiving ciclosporin rather than azathioprine (with a mean interval of 48 months) • The bone marrow, liver and lung are often affected Differentiating between Hodgkin’s disease and Non-Hodgkin’s lymphoma
Lymphoma
LYMPHOMA
INTRODUCTION
DEFINITION
 if bone marrow or peripheral blood involvement predominates it is then known as a leukaemia
if bone marrow or peripheral blood involvement predominates it is then known as a leukaemia
Hodgkin’s disease (HD)
 it is subclassified into four histological types:
it is subclassified into four histological types:
 Lymphocyte-rich classical HD (5%): an often indolent disease occurring within peripheral lymph nodes
Lymphocyte-rich classical HD (5%): an often indolent disease occurring within peripheral lymph nodes
 Mixed cellularity (MC) classical HD (20–25%): this is more commonly seen in males and is associated with B symptoms
Mixed cellularity (MC) classical HD (20–25%): this is more commonly seen in males and is associated with B symptoms
 Nodular sclerosing (NS) classical HD (70%): many fibrotic bands are present
Nodular sclerosing (NS) classical HD (70%): many fibrotic bands are present  it typically presents in young females as a bulky mediastinal or neck mass
it typically presents in young females as a bulky mediastinal or neck mass
 Lymphocyte-depleted (LD) classical HD (< 5%): this is seen with HIV-associated disease
Lymphocyte-depleted (LD) classical HD (< 5%): this is seen with HIV-associated disease
CLINICAL PRESENTATION
Systemic ‘B’ symptoms (up to 40%)
 drenching night sweats
drenching night sweats  weight loss (>10% of the patients body weight)
weight loss (>10% of the patients body weight)
NHL
 immunosuppression is an important aetiological factor, with a high incidence in AIDS patients and those on long-term immunosuprression
immunosuppression is an important aetiological factor, with a high incidence in AIDS patients and those on long-term immunosuprression
 whilst the incidence of HD has remained stable, that of NHL has risen significantly (which is explained in part by the use of new classification techniques and also as a consequence of the increased incidence of NHL associated with immune deficiencies)
whilst the incidence of HD has remained stable, that of NHL has risen significantly (which is explained in part by the use of new classification techniques and also as a consequence of the increased incidence of NHL associated with immune deficiencies)
AETIOLOGY
Infectious agents
 it is also an important trigger for lymphomas occurring in congenital immunodeficiencies, immunosuppressed organ transplant patients and patients receiving chemotherapy
it is also an important trigger for lymphomas occurring in congenital immunodeficiencies, immunosuppressed organ transplant patients and patients receiving chemotherapy
STAGING
PROGNOSIS
TREATMENT
HD
 A large mediastinal mass (i.e. > ⅓ of the intrathoracic diameter at the level of T5) is generally treated with a moderate amount of initial chemotherapy in order to shrink the mass prior to any subsequent radiotherapy – this aims to avoid any excessive irradiation of the lung parenchyma and subsequent radiation fibrosis
A large mediastinal mass (i.e. > ⅓ of the intrathoracic diameter at the level of T5) is generally treated with a moderate amount of initial chemotherapy in order to shrink the mass prior to any subsequent radiotherapy – this aims to avoid any excessive irradiation of the lung parenchyma and subsequent radiation fibrosis
 although HD is highly radiosensitive there is risk of secondary cancers (e.g. thyroid and breast) within the area of the mantle radiotherapy field
although HD is highly radiosensitive there is risk of secondary cancers (e.g. thyroid and breast) within the area of the mantle radiotherapy field
NHL
 radiotherapy alone is considered for the small proportion of patients with stage I disease and no adverse factors (in whom surgical excision alone is considered inappropriate)
radiotherapy alone is considered for the small proportion of patients with stage I disease and no adverse factors (in whom surgical excision alone is considered inappropriate)
BURKITT’S LYMPHOMA
DEFINITION
 it is associated with immunodeficiency (usually HIV)
it is associated with immunodeficiency (usually HIV)
CLINICAL PRESENTATION
 mean age 7 years (2–16 years)
mean age 7 years (2–16 years)
PEARLS
Stage
Classification
I
Involvement of a single lymph node region (I) or a single extralymphatic organ or site (IE)
II
Involvement of two or more lymph node regions on the same side of the diaphragm (II) or one or more lymph node regions plus an extralymphatic site (IIE)
III
Involvement of lymph node regions on both sides of the diaphragm (III) (the spleen is included in stage III)  subdivided into:
subdivided into:
IV
Involvement of one or more extralymphatic organs (e.g. lung, liver, bone, bone marrow) with or without lymph node involvement
Additional qualifiers denote the following:
A: asymptomatic
B: fever, night sweats and weight loss of >10% body weight
X: bulky disease (defined as a lymph node mass >10cm in diameter or, if involving the mediastinum, a mass greater than ⅓ of the intrathoracic diameter at the level of T5)
E: involvement of a single extranodal site, contiguous with a known nodal site
LYMPH NODE DISEASE IN LYMPHOMA
SPECIFIC FORMS OF LYMPHOMA
MUCOSA-ASSOCIATED LYMPHOID TISSUE (MALT) LYMPHOMAS
 Hashimoto’s thyroiditis: a 70× increased risk of thyroid lymphoma
Hashimoto’s thyroiditis: a 70× increased risk of thyroid lymphoma
 Sjögren’s syndrome: a 44× risk of lymphoma
Sjögren’s syndrome: a 44× risk of lymphoma
 most patients present with stage IE or IIE disease, which tends to be indolent
most patients present with stage IE or IIE disease, which tends to be indolent
 the small bowel and colon are involved in immunoproliferative small intestinal disease (IPSID), which was previously known as alpha-chain disease
the small bowel and colon are involved in immunoproliferative small intestinal disease (IPSID), which was previously known as alpha-chain disease  bone marrow involvement is seen in 20%
bone marrow involvement is seen in 20%  other sites of involvement include the lungs, head, neck, ocular adnexae, skin, thyroid and breast
other sites of involvement include the lungs, head, neck, ocular adnexae, skin, thyroid and breast
 Multiple extranodal sites are involved in 10% but this does not appear to have the same poor prognostic import as other forms of NHL
Multiple extranodal sites are involved in 10% but this does not appear to have the same poor prognostic import as other forms of NHL
 Up to 20% of lymphomas involving Waldeyer’s ring are of the MALT type with the tonsils the most commonly affected
Up to 20% of lymphomas involving Waldeyer’s ring are of the MALT type with the tonsils the most commonly affected  the commonest pattern is asymmetrical thickening of the pharyngeal mucosa
the commonest pattern is asymmetrical thickening of the pharyngeal mucosa
LYMPHOMA IN THE IMMUNOCOMPROMISED
Lymphomas associated with HIV
 EBV positivity occurs in up to 70% of patients
EBV positivity occurs in up to 70% of patients
 Various types are seen, including those in immunocompetent patients such as BL and DLBCL, but some occur more frequently in HIV patients (e.g. primary effusion lymphoma and plasmablastic lymphoma of the oral cavity)
Various types are seen, including those in immunocompetent patients such as BL and DLBCL, but some occur more frequently in HIV patients (e.g. primary effusion lymphoma and plasmablastic lymphoma of the oral cavity)  DLBCL tends to occur later, whereas BL occurs in less immunodeficient patients
DLBCL tends to occur later, whereas BL occurs in less immunodeficient patients
 there is a marked propensity to involve extranodal sites (especially the GI tract, CNS, liver and bone marrow)
there is a marked propensity to involve extranodal sites (especially the GI tract, CNS, liver and bone marrow)  multiple sites of extranodal involvement are common (> 75%)
multiple sites of extranodal involvement are common (> 75%)  peripheral lymph node enlargement is relatively uncommon
peripheral lymph node enlargement is relatively uncommon
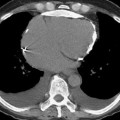
 pleural effusions, nodules, acinar and interstitial opacities are common
pleural effusions, nodules, acinar and interstitial opacities are common  hilar and mediastinal nodal enlargement is generally mild
hilar and mediastinal nodal enlargement is generally mild
 Abdomen: the GI tract, liver, kidneys, adrenal glands and lower GU tract are commonly involved
Abdomen: the GI tract, liver, kidneys, adrenal glands and lower GU tract are commonly involved  imaging appearances are similar to those seen in immunocompetent patients (although mesenteric and retroperitoneal nodal enlargement is less common)
imaging appearances are similar to those seen in immunocompetent patients (although mesenteric and retroperitoneal nodal enlargement is less common)
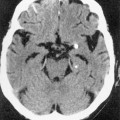
 rim enhancement and multifocality are seen more often than in the immunocompetent population (causing confusion with cerebral toxoplasmosis although the location of PCNSL within the deep white matter is suggestive)
rim enhancement and multifocality are seen more often than in the immunocompetent population (causing confusion with cerebral toxoplasmosis although the location of PCNSL within the deep white matter is suggestive)
Post-transplant lymphoproliferative disorders (PTLD)
 marrow allograft recipients in general have a low risk (1%)
marrow allograft recipients in general have a low risk (1%)
 The lowest frequency is seen in renal transplant recipients (1%)
The lowest frequency is seen in renal transplant recipients (1%)  the highest frequency is seen in heart–lung or liver–bowel allografts (5%)
the highest frequency is seen in heart–lung or liver–bowel allografts (5%)
 EBV-positive cases occur earlier than EBV-negative cases (the latter occurring 4–5 years after transplantation)
EBV-positive cases occur earlier than EBV-negative cases (the latter occurring 4–5 years after transplantation)  in all cases, extranodal disease is disproportionately commoner
in all cases, extranodal disease is disproportionately commoner
 In patients receiving azathioprine, the allograft itself and the CNS are often involved (in patients who have received ciclosporin the GI tract is affected more than the CNS)
In patients receiving azathioprine, the allograft itself and the CNS are often involved (in patients who have received ciclosporin the GI tract is affected more than the CNS)
 multiple intrapulmonary masses, pleural effusions, and involvement of multiple segments of bowel and the transplanted organ have all been reported
multiple intrapulmonary masses, pleural effusions, and involvement of multiple segments of bowel and the transplanted organ have all been reported
 the majority (> 90%) are B-cell lymphomas
the majority (> 90%) are B-cell lymphomas axillary (up to 20%)
axillary (up to 20%)  inguinal (up to 15%)
inguinal (up to 15%)  hepatosplenomegaly
hepatosplenomegaly
 fatigue
fatigue  anorexia
anorexia  rarely alcohol-induced pain at the site of any enlarged lymph nodes
rarely alcohol-induced pain at the site of any enlarged lymph nodes in severe immunodeficiency states (e.g. AIDS and with organ transplantation) lymphomas are often EBV-driven large B-cell lymphomas
in severe immunodeficiency states (e.g. AIDS and with organ transplantation) lymphomas are often EBV-driven large B-cell lymphomas there is also a slight male preponderance for HD and NHL
there is also a slight male preponderance for HD and NHL

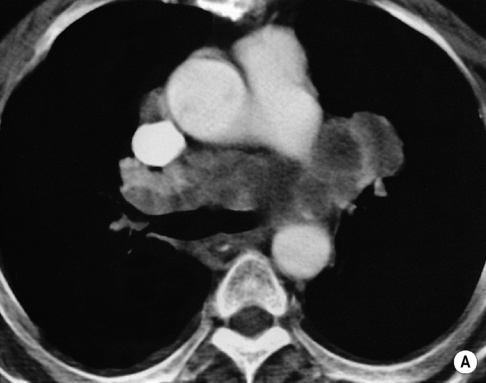
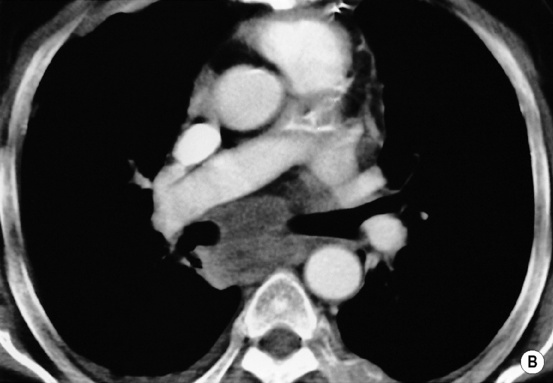
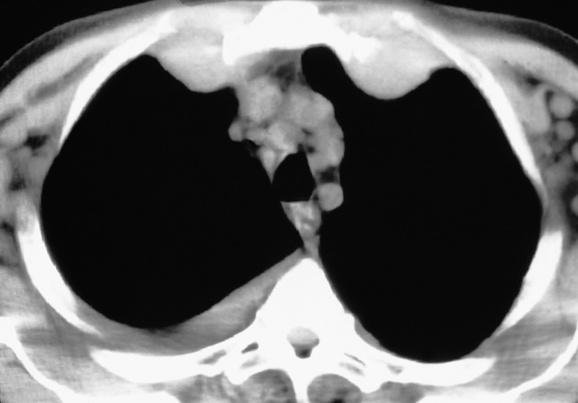
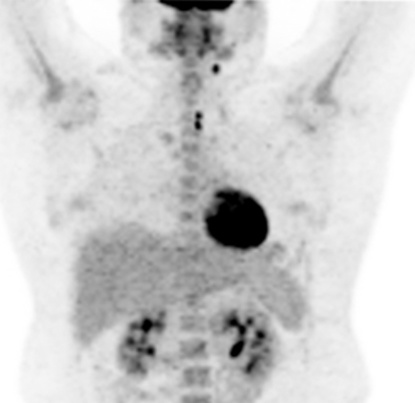






 high-grade tumours carry a worse prognosis but are potentially curable
high-grade tumours carry a worse prognosis but are potentially curable




 the ovaries, kidneys and breast may also be affected
the ovaries, kidneys and breast may also be affected there is a similar appearance in other bones
there is a similar appearance in other bones the ovaries, kidneys and breasts are commonly involved
the ovaries, kidneys and breasts are commonly involved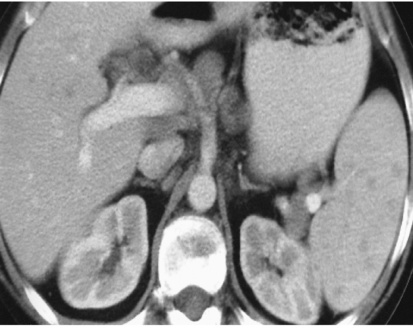
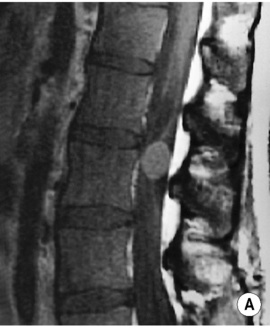
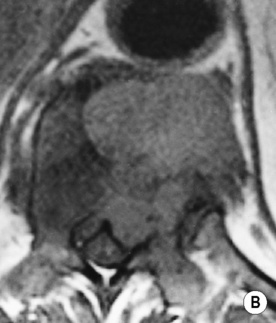
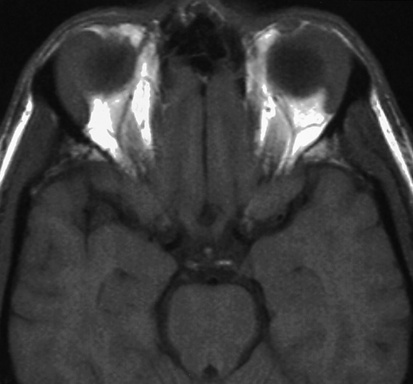
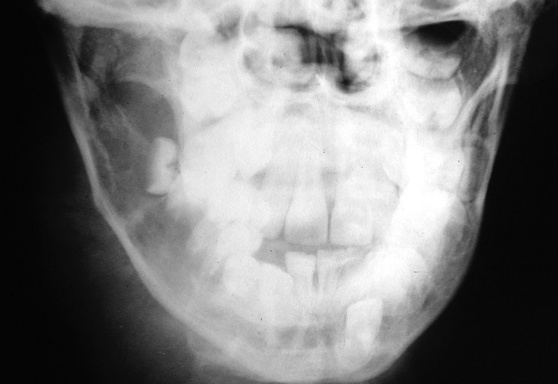





 involved nodes tend to be larger than in HD
involved nodes tend to be larger than in HD gastrohepatic ligament/porta hepatis nodes > 8mm
gastrohepatic ligament/porta hepatis nodes > 8mm  retrocrural nodes > 6mm
retrocrural nodes > 6mm  supraclavicular nodes > 5mm
supraclavicular nodes > 5mm  pelvic nodes > 8mm
pelvic nodes > 8mm  any nodes seen at the splenic hilum, or presacral and perirectal areas are considered unusual
any nodes seen at the splenic hilum, or presacral and perirectal areas are considered unusual T2WI: intermediate-to-high SI
T2WI: intermediate-to-high SI  STIR: very high SI
STIR: very high SI supraclaviclar or bilateral neck adenopathy is associated with an increased risk of infradiaphragmatic disease
supraclaviclar or bilateral neck adenopathy is associated with an increased risk of infradiaphragmatic disease 40–60% of patients presenting with head and neck involvement will have disseminated NHL
40–60% of patients presenting with head and neck involvement will have disseminated NHL the paracardiac nodes are an important site of recurrence as they are not included in the classical ‘mantle’ radiation field
the paracardiac nodes are an important site of recurrence as they are not included in the classical ‘mantle’ radiation field they can be discrete or matted together with cystic changes seen within large anterior mediastinal masses
they can be discrete or matted together with cystic changes seen within large anterior mediastinal masses splenic hilar involvement is almost always associated with diffuse splenic infiltration
splenic hilar involvement is almost always associated with diffuse splenic infiltration
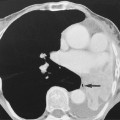
 regional nodal involvement is frequently seen with primary extranodal lymphoma involving an abdominal viscus
regional nodal involvement is frequently seen with primary extranodal lymphoma involving an abdominal viscus the presence of central necrosis or multilocular enhancement suggests an alternative diagnosis (e.g. tuberculosis or atypical infection)
the presence of central necrosis or multilocular enhancement suggests an alternative diagnosis (e.g. tuberculosis or atypical infection) inguinal or femoral adenopathy is seen in < 20% of HD patients at presentation
inguinal or femoral adenopathy is seen in < 20% of HD patients at presentation