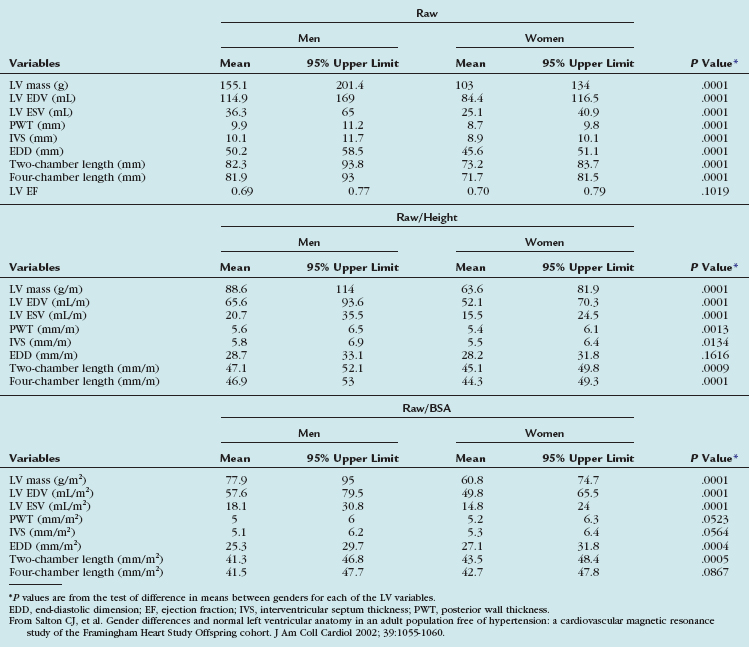
TABLE 55-3 Means and 95% Upper Limits for Raw, Height-Adjusted, and Body Surface Area–Adjusted Left Ventricular Variables
CHAPTER 55 Magnetic Resonance and Computed Tomographic Imaging of Myocardial Function
The work-up of ischemic heart disease necessitates an accurate and reproducible method of assessing cardiac function. The importance of cardiac function analysis in the evaluation of coronary artery disease (CAD) lies in the diagnostic and prognostic implications and, consequently, its usefulness in guiding medical therapy. Being the reference standard technique, MRI can quantify global systolic function and detect regional abnormalities in wall motion and help establish the diagnosis of CAD. In addition, pharmacologic stress imaging protocols using function or perfusion MRI have been shown to provide a sensitive detection of CAD.1 CT seems promising in at least some of these applications,2 although it is currently limited by the temporal resolution and scarce clinical validation data. Exercise stress imaging using MRI has been performed, although its feasibility in assessing patients with or suspected to have ischemic heart disease has been shown only in select, highly experienced sites.3
Prognosis after myocardial infarction (MI) has been correlated with the extent of myocardial necrosis and the degree of contractile dysfunction of the left ventricle.4 In patients with MI and chronic left ventricular (LV) dysfunction, LV ejection fraction and LV volumes are important prognostic parameters.5 The extent of late myocardial hyperenhancement on MRI and regional contractile improvement in response to low-dose dobutamine stress MRI can accurately predict recovery of function after MI, identify myocardial viability, and effectively guide coronary revascularization therapy.6–9
Although still experimental, growing evidence suggests that assessment of diastolic functional parameters is possible using current CT and MRI techniques. A time/volume curve per heart cycle is calculated on multidetector CT, from which determination of the peak filling rate (PFR) and the ratio of time to peak filling rate to R–R interval (tPFR/RR) have been evaluated as parameters of LV diastolic function. The early-to-late diastolic filling ratio (E/A) on Doppler echocardiography has been found to correlate positively with PFR (r = 0.54) and negatively with tPFR/RR (r = −0.57) on multidetector CT.10 Using MRI, a time/volume curve can likewise be derived, and diastolic function is expressed as PFR, early and active PFR (PFRE and PFRA), and their ratios. Diastolic parameters using echocardiography and MRI have been shown to vary by gender and age. Aging results in a decrease in LV distensibility that increases the early diastolic filling time, allowing the ventricle more time to fill, and increases the contribution of the atrial kick to LV filling. Although Doppler echocardiography provides peak velocities, MRI provides absolute peak filling rates from the time/volume curves. These data are also obtainable from radionuclide ventriculography; however, MRI has the advantage of significantly higher spatial resolution.11
Another more advanced MRI technique that has been studied in the evaluation of diastolic function involves the use of a high temporal resolution velocity-mapping technique that is able to identify previously undetectable myocardial motion patterns. This method has been shown to be comparable to tissue Doppler imaging—a well-established method that allows noninvasive assessment of diastolic function. Tissue Doppler imaging is limited, however, by limitations in acoustic window, low velocity resolution, lack of spatial information, and effect of the angle of insonation on myocardial velocities. Other established MRI methods for quantifying wall motion, such as tagging, phase contrast velocity mapping (tissue phase mapping), and displacement encoding with stimulated echoes (DENSE), can also be used to extract parameters such as strain or radial and circumferential velocity components, which are measures of diastolic function.12
Various imaging modalities are available to assess ventricular systolic function. Direct left ventriculography may be used, but it is rarely employed for the sole purpose of determining LV function because of its invasive nature. Among noninvasive imaging modalities, echocardiography is the most widely used because of its ease, relatively low cost, and widespread availability. Nuclear scintigraphy including gated single photon emission computed tomography (SPECT) and positron emission tomography can assess global and regional LV function, although their accuracies in this regard have been challenged by low spatial and temporal resolution. Radionuclide ventriculography provides accurate and reproducible assessment of global LV function. More recently, multidetector CT has been used in the evaluation of global and regional systolic LV function.13,14 Among all noninvasive imaging modalities, MRI has been shown to be the most accurate and reproducible modality for the evaluation of global and regional systolic LV function.15
INDICATIONS AND APPLICATIONS OF MAGNETIC RESONANCE IMAGING AND COMPUTED TOMOGRAPHY IN THE EVALUATION OF ISCHEMIC HEART DISEASE
Magnetic Resonance Imaging
Improvements in MRI have introduced new methods for evaluating CAD and its consequences. MRI often provides additional data that may be unavailable from other noninvasive imaging modalities, such as echocardiography, nuclear scintigraphy, and CT. Table 55-1 lists indications for MRI in CAD based on the European Consensus Panel report. Not included in this list are less common techniques, such as T2* measurements and T2-weighted edema imaging, which are being increasingly used in more experienced centers for assessment of cardiomyopathy of unknown etiology.
TABLE 55-1 Indications for Magnetic Resonance Imaging in Coronary Artery Disease
| Indication | Class* |
|---|---|
| Assessment of Global Ventricular (Left and Right) Function and Mass | I |
| Detection of CAD | |
| Regional LV function at rest and during dobutamine stress | II |
| Assessment of myocardial perfusion | II |
| Coronary MR angiography (CAD) | III |
| Coronary MR angiography (anomalies) | I |
| Coronary MR angiography of bypass graft patency | II |
| MRI flow measurements in coronary arteries | Inv |
| Arterial wall imaging | Inv |
| Acute and Chronic MI | |
| Detection and assessment | I |
| Myocardial viability | I |
| Ventricular septal defect | III |
| Mitral regurgitation (acute MI) | III |
| Ventricular thrombus | II |
| Acute coronary syndromes | Inv |
* Indication class: I, provides clinically relevant information and is usually appropriate; may be used as first-line imaging technique; usually supported by substantial literature; II, provides clinically relevant information and is frequently useful; other techniques may provide similar information; supported by limited literature; III, provides clinically relevant information, but is infrequently used because information from other imaging techniques is usually adequate; Inv, potentially useful, but still investigational.
From Pennell DJ, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J 2004; 25:1940-1965.
Computed Tomography
CT is increasingly being used in diagnosis of CAD. Because of its prognostic implications, the quantification of coronary artery calcification is a growing clinical application. Arad and colleagues17 were the first to report attempts to predict cardiac events with coronary calcium as detected by electron-beam CT, showing increased risk of cardiovascular events with higher Agatston scores. Coronary calcium scoring is discussed in Chapter 32.
Contrast-enhanced CT angiography of the coronary arteries is another application of multidetector CT used in the delineation of coronary anatomy and the detection of vascular stenosis. This technique has been used in the detection of CAD in patients at risk, and has been found to correlate with findings on invasive angiography.18
Multidetector CT was initially primarily used for detection of coronary artery stenosis and assessment of cardiac morphology, but more recently has been shown to be useful for cardiac function.10,13,14 Considering the need for administration of contrast material, radiation exposure, and limited temporal resolution (80 to 250 ms), use of multidetector CT exclusively for analysis of cardiac function parameters does not seem clinically prudent at present. If the data are already obtained across all phases of the cardiac cycle during a standard coronary CT angiography evaluation, however, the combination of noninvasive coronary artery imaging and assessment of cardiac function with multidetector CT is a suitable approach because it enables a more comprehensive cardiac work-up in patients with suspected CAD.
Quantification of cardiac function using multidetector CT has been shown to be in good agreement with echocardiography, invasive cine ventriculography, SPECT, and MRI as the reference standard.19 In addition, CT provides a useful alternative to MRI in the evaluation of ischemic heart disease in patients in whom MRI is contraindicated (e.g., patient has a periorbital metallic foreign body or metallic device; see Chapter 19 on MR safety for more information). Table 55-2 compares the advantages and disadvantages of the use of CT versus MRI in the evaluation of CAD.
TABLE 55-2 Advantages and Disadvantages of Magnetic Resonance Imaging and Computed Tomography
| MRI | Multidetector CT | |
|---|---|---|
| Advantages | No ionizing radiation | Widely available |
| No exposure to iodinated contrast media | Ease and rapidity of performance | |
| Better temporal and spatial resolution | Not contraindicated in patients with cardiac devices | |
| Better validation in clinical assessment of the following: | Single breath-hold acquisition eliminates motion artifact | |
| Better quality of images in the assessment of coronary artery anatomy | ||
| Can be used clinically to assess the following (although with less validation studies compared with MRI): | ||
| Ventricular mass, volumes and global systolic function | ||
| Regional myocardial function | ||
| MI | ||
| Disadvantages | Contraindicated in patients with noncompatible biometallic implants, pacemakers or implantable cardioverter defibrillators | Valvular function |
| Diastolic function | ||
| Stress testing and assessment of myocardial perfusion-ischemia possible, but clinical experience is scant | ||
| Radiation exposure | ||
| Risk of contrast medium allergy | ||
| Risk of worsening renal function from iodinated contrast dye exposure |
ASSESSMENT OF CARDIAC FUNCTION
Global Systolic Function
Magnetic Resonance Imaging
Anatomic Planning and Standard Views
To produce high accuracy and reproducibility, standard imaging planes need to be used in quantifying ventricular function. The axes of the heart are defined using transverse, coronal, and sagittal views obtained as three-plane or individual scout images or through the use of real-time imaging techniques. We present one of many approaches to localize quickly and obtain the standard imaging planes for this purpose using cine MRI. Coronal images are used to define the transverse mid-ventricular slices (i.e., oblique axial images or “quasi” four-chamber view*). The short-axis views of the left ventricle can be defined by prescribing views perpendicular to the line between the center of the mitral valve and apex on the “quasi” four-chamber view. Slices are obtained from the base of the left ventricle at the level of the mitral annulus to the apex, allowing for full LV coverage for proper calculation of LV volumes, ejection fraction, and mass.
RV function is typically evaluated using the previously described LV views to limit total imaging time. A more detailed evaluation of RV structure and function, of particular importance in the evaluation of arrhythmogenic RV dysplasia, requires the acquisition of additional views. Standard transverse views from cranial to caudal slices are roughly perpendicular to the RV outflow tract, RV free wall, and RV inflow. To visualize the diaphragmatic segment of the right ventricle, a slice planned on a coronal scout, generating an oblique sagittal view through the long axis of the RV outflow tract, should be acquired.20
Sequences
Evaluation of cardiac function involves the use of multiphase, or cine, MRI that employs fast gradient-echo imaging with k-space segmentation to acquire images during end-inspiration breath-holding, which minimizes respiratory motion artifact.21 It is generally accepted that a temporal resolution of 50 ms is required to “freeze” end-systolic motion accurately to quantify ventricular volumes and calculate ejection fraction accurately. Segmented k-space breath-hold cine imaging has an in-plane spatial resolution that typically ranges from 1.5 to 2 mm2, slice thickness of 5 to 10 mm, temporal resolution of 30 to 50 ms, and scan times of 8 to 16 s/slice. The k-space segmentation allows for shorter acquisition times and enables the acquisition of a complete set of multiphase images per slice within a breath-hold. Generally, blood appears bright and myocardium appears gray in these cine gradient-echo images; however, the specific appearance of blood, myocardium, and blood flow depends on whether spoiled gradient-recalled-echo or steady-state free precession (SSFP) is used for signal generation within the segmented k-space structure.15
The use of spoiler gradients is the most commonly employed method, and involves extending the duration of the readout gradient beyond the sampling of the echo and before the next RF pulse. The prolonged application of the gradient results in accelerated dephasing of the residual transverse magnetization.22 Because residual magnetization is spoiled, signal intensity is not maximized. To improve image quality, SSFP imaging of the heart was later developed, which offers specific advantages to cardiac imaging. This method results in improved signal-to-noise and contrast-to-noise ratios.23 With SSFP, residual transverse magnetization is refocused, not spoiled, before the next RF pulse, and signal intensity is maximized. With this method, magnetization is in a steady state, so the RF pulses are applied throughout the acquisition.
SSFP is extremely sensitive to field inhomogeneities, of particular importance at 3.0 T, where repeated local shimming or frequency manipulation may be necessary to improve image quality. Traditional fast gradient-echo pulse sequences should be used for the detection of subtle flow abnormalities in valvular heart disease, or when field inhomogeneities result in significant artifacts with SSFP.22 The energy deposition associated with continuous application of RF pulses to maintain steady-state magnetization is higher, and specific absorption rate limits may be reached during SSFP, particularly with higher performance gradient scanners.
Myocardial contractility and regional function can be evaluated further through the use of cine MRI with tagging in conjunction with segmented k-space cine gradient-echo imaging, also known as spatial modulation of magnetization. To evaluate diastolic function, the tagging pulse may be applied in late systole, with images acquired throughout diastole. Quantification of wall motion and myocardial strain can be determined based on displacement of the tagged regions.22 To overcome the time-consuming aspect of tag analysis, harmonic phase analysis and DENSE techniques have been developed to improve the postprocessing duration.24–26
Image Analysis
Quantification of myocardial mass, ventricular volumes and dimensions, stroke volume, and ejection fraction is currently done using volumetric data computed from tracings of contiguous short-axis images obtained using cine SSFP pulse sequences. It is important to identify correctly the most basal extent of the ventricle because this determines the LV volumes and the LV ejection fraction.27 Manual planimetry of LV endocardial and epicardial borders is performed during end-systole and end-diastole on each of the short-axis slices (Fig. 55-1). Because of the time required to do manual planimetry, automatic and semiautomatic methods have been developed. In studies comparing manual, automatic, and semiautomatic techniques in animals that were sacrificed for ex vivo measurements, it was determined that manual contours were closer to ex vivo measurements than automatic contours, however, which overestimates true volume.28,29 After border detection, cardiac volumes are calculated by multiplying the blood pool area (defined by the endocardial border) by the slice thickness and summing all the slices. Myocardial mass is computed from the difference between the volumes determined from the endocardial and epicardial borders multiplied by the specific gravity of the myocardium (1.05 g/cm3).15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



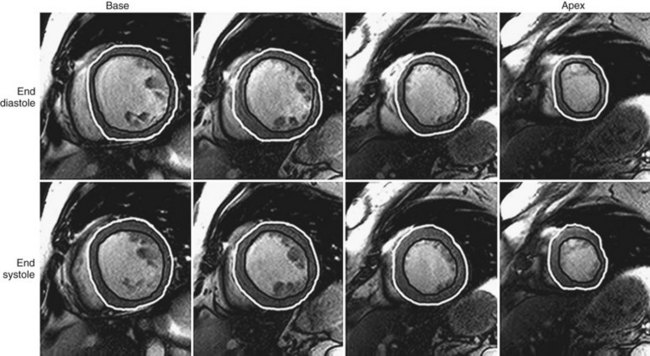
 FIGURE 55-1
FIGURE 55-1