CHAPTER 82 Magnetic Resonance Angiography
Clinical Techniques
DESCRIPTION OF TECHNICAL REQUIREMENTS
Field Strength
It has been established that signal-to-noise ratio (SNR) increases in an approximately linear fashion with magnetic field strength, which provides the opportunity for immensely superior vascular depiction with imaging at 3.0 T compared with 1.5 T. High field strength imaging is associated with a realm of potential challenges, however, which are relatively less significant at 1.5 T, including specific absorption rate considerations, T2* and dielectric resonance effects, and a greater incidence of clinically appreciable peripheral nerve stimulation.1 Use of a 3.0 T system demands familiarity with methods of avoiding and addressing these challenges such that compromised patient safety or image quality is not acceptable. Although higher field strength has advantages for MR angiography, it is not essential, and high-quality diagnostic examinations are routinely produced on 1.5 T MRI systems.
Gradient Coils
More recent developments in MRI hardware design have facilitated further improvements in gradient coil performance.2 High-performance gradient coils enable optimization of vascular SNR. These SNR improvements incur a penalty, however, in the form of energy deposition and increases in specific absorption rate. In practical terms, limitations in specific absorption rate often necessitate a compromise in attainable slice coverage for a particular repetition time (TR).
Phased-Array Surface Coils and Parallel Imaging Techniques
Comprising multiple integrated receiver coils, phased-array coils combine the advantages of high SNR achieved by smaller coils with the benefits of improved volume coverage, previously afforded only by large coil elements. Parallel imaging techniques (e.g., sensitivity encoding, or SENSE),3 whereby incomplete k-space sampling is tolerated by coil sensitivity profile calculation of the missing data, allow for significant improvements in temporal resolution, spatial resolution, or volume coverage. Parallel imaging depends on phased-array surface coils for its application. Parallel imaging improvements are attained at the expense, however, of reduced SNR. Such SNR loss may be offset, and even reversed, by imaging at higher field strengths (e.g., 3.0 T), allowing the benefit of ever-increasing acceleration factors to be realized without compromise in field of view (FOV) or spatial resolution.4
TIME OF FLIGHT MAGNETIC RESONANCE ANGIOGRAPHY
Repetitive successive radiofrequency pulses, if applied at a magnitude and rate sufficient to prevent interval T1 recovery, results in saturation of signal from tissue within the imaged volume.5 Time of flight (TOF) MR angiography exploits this saturation effect, providing untainted visualization of the signal produced by unsaturated entry of blood (i.e., through-plane blood flow) without the requirement for contrast agent administration. Unidirectional flow may be imaged through the use of presaturation pulses (also known as saturation bands) to eliminate signal from spins traveling in the opposite direction, with the effect of providing pure angiographic or venographic depiction, as desired. These attributes have made TOF MR angiography the most established MR angiography technique currently available, particularly with regard to the carotid, vertebral, and intracranial vascular territories.
Numerous potential implementations of this technique are available. Each varies in its degree of suitability, depending on the clinical indication. Two-dimensional TOF MR angiography involves the excitation of a single anatomic section and has proven useful for the evaluation of anatomic regions where respiratory or cardiac motion precludes useful volumetric evaluation (e.g., chest or abdomen). Multiple breath-holds and sequential, independent two-dimensional TOF MR angiography acquisitions may be used in this instance to provide diagnostic quality examinations, even in dyspneic patients (Fig. 82-1). Three-dimensional TOF MR angiography is preferred for intracranial evaluation in particular, permitting detailed volumetric data acquisition at submillimeter voxel resolution and the potential for subsequent postprocessing (Fig. 82-2). Multiple overlapping thin slab acquisition (MOTSA) represents a compromise in two-dimensional and three-dimensional techniques, integrating the advantages of three-dimensional imaging with the relatively fewer limitations of the two-dimensional approach. MOTSA combines multiple, relatively thin three-dimensional slabs to provide clinically useful volume coverage.6
Indications
Before the widespread introduction of contrast-enhanced MR angiography techniques for comprehensive large-volume anatomic vascular coverage, TOF MR angiography represented the cornerstone of MR angiography throughout the body. Contrast-enhanced MR angiography, however, required revision of many diagnostic algorithms in favor of this faster and typically higher quality method. Nonetheless, TOF MR angiography remains the technique of choice for intracranial vascular depiction, a reflection of its superb spatial and contrast resolution and its patient acceptability.7 Advances in MRI hardware, including the introduction of dedicated head and neck coils and resultant implementation of parallel imaging techniques, have enhanced the value of this approach in clinical practice further.
Pitfalls and Solutions
Despite its popularity and widespread implementation, TOF MR angiography may be extremely challenging to implement and interpret because of its numerous potential pitfalls.8
Saturation
As explained, successful TOF MR angiography depends on saturation of signal from static tissue, such that “fresh” through-plane vascular spins produce an appreciable signal. Saturation of blood signal occurs if blood flow is slow or persists within the imaging field (e.g., vessel coursing in-plane) and can result in suboptimal vascular visualization, to the point of potentially mimicking a vascular occlusion. This situation is of particular significance with regard to the use of three-dimensional TOF MR angiography, owing to the more extensive volume coverage required for most body applications. Numerous potential solutions to this dilemma exist, including optimization of TR and imaging plane, reduction of flip angle and echo time (TE), and use of thinner slices. Three-dimensional TOF MOTSA provides the advantages of three-dimensional TOF MR angiography, but by using thinner three-dimensional slabs, minimizes the saturation effects over that of a single large three-dimensional volume. If saturation effects persist in small, slow-flow vessels, administration of a small amount of T1-shortening paramagnetic contrast agent may prove effective, although at the risk of inducing adjacent soft tissue enhancement and venous contamination.9
PHASE CONTRAST MAGNETIC RESONANCE ANGIOGRAPHY
Phase contrast MR angiography is an unenhanced approach to imaging that employs bipolar phase-encoding gradient pairs to encode flow velocity in the gradient direction. Stationary background tissue accumulates a net phase shift of zero. Moving spins experience a net phase shift that produces signal and the image contrast necessary to distinguish between moving and stationary tissue (i.e., angiography).10 Phase contrast MR angiography requires the operator selection of a velocity encoding (VENC) in cm/s, which is responsible for determination of the flow sensitivity of the acquisition. Because assignment of phase shift is limited to a range of −180 degrees to +180 degrees, the VENC represents a flow velocity that would cause a maximal phase shift of 180 degrees. For optimal sensitivity, this VENC should be selected to correspond with or slightly exceed the highest velocity present within the vessel in question. For intracranial applications, a VENC of 70 to 80 cm/s is often sufficient for arterial imaging, whereas a factor of 20 to 30 cm/s should be applied for venous imaging.11 If the flow velocity exceeds the chosen VENC, aliasing results with the effect of apparent flow reversal.
Indications
Before the widespread availability of contrast-enhanced and increasingly impressive TOF techniques, phase contrast MR angiography was relatively successful in the evaluation of various vascular territories. In recent times, this approach has been relegated in importance to that of a “last resort,” should the other angiographic techniques discussed in this chapter be unsuccessful or contraindicated. Phase contrast MR angiography has regained some of its former popularity more recently because of its flow quantification capabilities. Our experience suggests that phase contrast flow quantification is a valuable, versatile tool in the noninvasive evaluation of flow characteristics within almost any vascular bed.12 Although this technique has not yet been incorporated into widespread clinical practice, its future potential remains encouraging.
THREE-DIMENSIONAL STEADY-STATE FREE PRECESSION MAGNETIC RESONANCE ANGIOGRAPHY
SSFP is a low flip angle gradient-recalled-echo (GRE) technique that induces a persistent level of tissue magnetization by means of a TR that is significantly shorter than the T2 of tissue. As a result, this approach permits bright blood vascular imaging, the signal from which is a reflection of the inherent T2/T1 ratio of blood, while precluding gadolinium-chelate contrast agent administration.13 Owing to a very short TR and large flip angle, two-dimensional SSFP techniques allow rapid subsecond image acquisition that does not require respiratory suspension, even when imaging the chest. These attributes have resulted in the adoption of SSFP as a cornerstone imaging technique in many aspects of cardiac imaging, including two-dimensional single-shot multiplanar morphologic and ECG gated cine functional myocardial assessment.
Many three-dimensional implementations of SSFP have been successfully evaluated for the purpose of vascular imaging, most notably with regard to the coronary and renal arteries.14,15 In exploiting the intrinsic T2/T1 signal of blood, three-dimensional SSFP MR angiography allows large FOV vascular coverage, while avoiding the data acquisition constraints because of the contrast bolus imposed during contrast-enhanced MR angiography. Combining three-dimensional SSFP MR angiography with navigator gating allows free-breathing nonenhanced chest and abdominal vascular depiction. Further addition of ECG gating has allowed the realization of free-breathing coronary MR angiography, although at the expense of often prolonged acquisition times (≥10 minutes) (Fig. 82-3). The potential of parallel imaging techniques to aid in reduction of these acquisition times has been evaluated, providing encouraging results to date. Implementation of this data-sharing technique does incur penalties with regard to SNR, however, with the effect of image degradation that may be poorly tolerated.
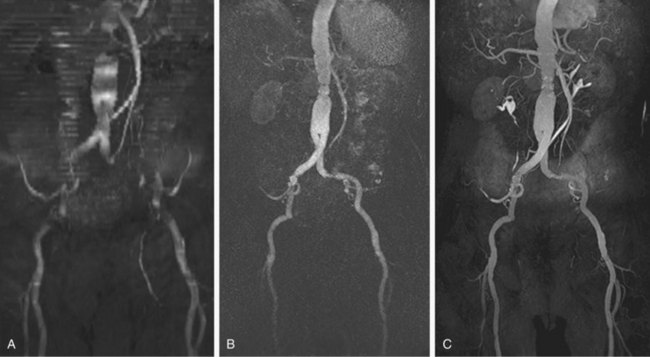
 FIGURE 82-1
FIGURE 82-1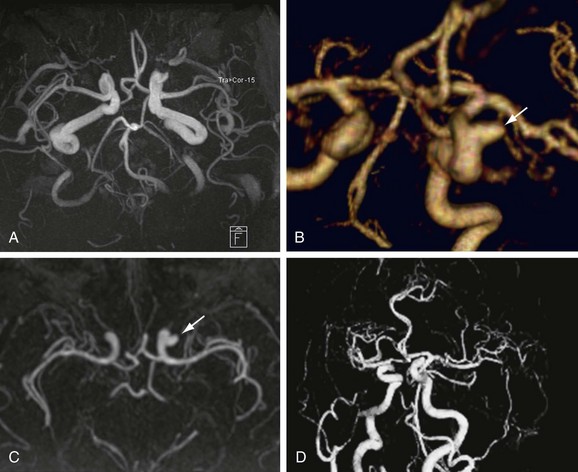
 FIGURE 82-2
FIGURE 82-2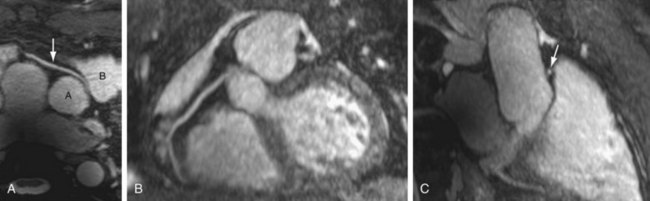
 FIGURE 82-3
FIGURE 82-3


