Colonoscopy remains the first and, in most cases, the sole investigation for detection and prevention of colorectal cancer. However, in up to 26% of examinations, the ileocecal valve cannot be reached by the endoscopist because of stenotic lesions or elongated colonic segments. Although computed tomography colonography (CTC; see Chapter 53 ) has received great appreciation in the last decade as an alternative colonic cancer screening examination, with a high accuracy for detection of polypoid lesions 1 cm or larger, radiation exposure remains a concern, especially for young patients with colonic pathology who require regular follow-up. In this context, the introduction of magnetic resonance colonography (MRC) holds great promise. The technique is based on the acquisition of MR datasets of the abdomen, with a distinct focus on the large bowel. Because of the noninvasive character and lack of procedural pain and discomfort, patient acceptance is improved compared with colonoscopy. The datasets can be displayed in a multiplanar reformation (MPR) mode on a postprocessing workstation, which enables the analysis of the colonic wall from any desired angle. In addition, virtual endoscopic views can be generated, so lesions may be more accurately defined. Concurrently with the colonic wall evaluation on MRC images, all abdominal organs within the displayed field of view can be also assessed.
Prerequisites and Examination Guidelines
General contraindications to MR imaging (MRI), including the presence of a pacemaker or metallic, non–MRI-compatible implants, have to be considered prior to MRC referral. In addition, patients with hip prostheses are not considered ideal candidates for MRC because significant artifacts predominantly obscure the anorectal region, downgrading image quality significantly.
A clean colon is required, especially when the clinical question to be answered is in regard to polyp detection. The cleansing method is usually the same, implemented locally for conventional colonoscopy or CTC.
Because most bowel loops are collapsed in their physiologic state, sufficient distention of the bowel is needed to optimize the examination. This allows for reliable differentiation between the bowel lumen and bowel wall. Most centers use water or barium solutions administered per rectum. The use of gaseous media has also been proposed, including room air or CO 2 . Prior to rectal filling, spasmolytic agents (e.g., 20 to 40 mg of scopolamine or 1 mg of glucagon) should be administered intravenously. The effect of these agents is threefold:
- 1.
A better degree of bowel distention can be achieved.
- 2.
Bowel spasm is minimized and thereby patient’s acceptance is enhanced.
- 3.
Absence of artifacts because bowel motion is reduced.
Patients are positioned in a prone or supine position on the scanner table. If liquid contrast agents are used for luminal distention, 2000 to 2500 mL is administered via a rectal tube, using hydrostatic pressure. The filling procedure should be stopped whenever patients express considerable discomfort. Alternatively, dedicated sequences can be acquired to monitor the filling process, such as nonslice select sequences providing an update image every 2 to 3 seconds. A combination of two large flex surface coils should be used for signal reception to ensure coverage of the entire large bowel. However, use of the body coil may be sufficient, especially for obese patients.
For data acquisition, the use of a 1.5-T scanner equipped with strong gradient systems is preferable. Data collection is mainly performed under breath-hold conditions. Thus, acquisition times of the single sequence should not exceed 20 to 25 seconds. Cardinal sequences used in MRC are the same as the ones used in MRI of the small bowel (MR enterography or enteroclysis; see Chapter 40 ). The acquisition of 2D and/or 3D fast imaging with steady-state precession (FISP) sequences has proven to be quite useful. Image properties are characterized by a mixture of T1 – weighted and T2 – weighted contrast, leading to a homogenous bright signal of the colonic lumen filled with water and providing good contrast with the colonic wall, which has low signal intensity. Furthermore, 3D T1 – weighted MRI scans should be acquired before and at 70 to 80 seconds after intravenous (IV) administration of gadolinium. Data collection is done in coronal and axial planes for all sequences described. Proposed MRC protocol sequence parameters are listed in Table 54-1 .
| Parameter | T1w VIBE | T1w FLASH | True FISP |
|---|---|---|---|
| 2D or 3D | 3D | 2D | 3D |
| Acquisition plane | Coronal | Axial | Coronal |
| Acquisition time (s) | 21 | 5 × 19 * | 22 |
| TR (ms) | 3.1 | 158 | 3.8 |
| TE (ms) | 1.1 | 1.8 | 1.9 |
| FLIP angle (degrees) | 12 | 70 | 80 |
| Slice thickness (mm) | 1.8 | 5.0 | 2.0 |
| No of slices | 120 | 70 | 96 |
Data Analysis
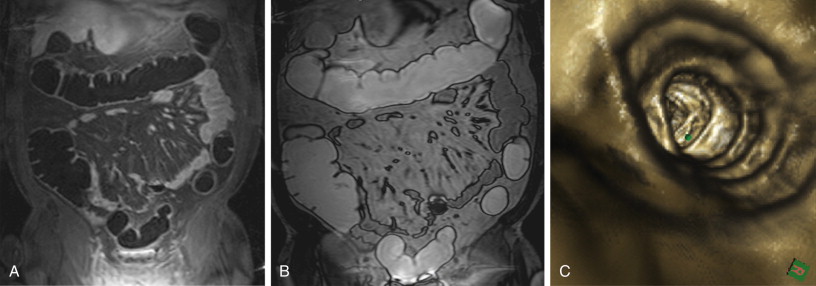
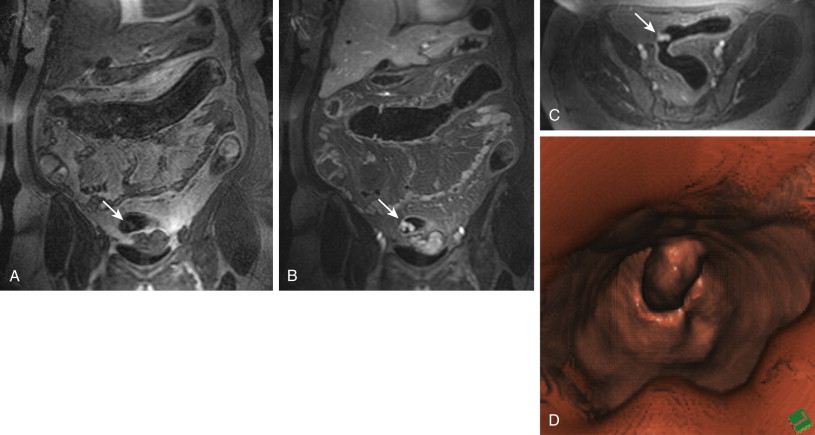
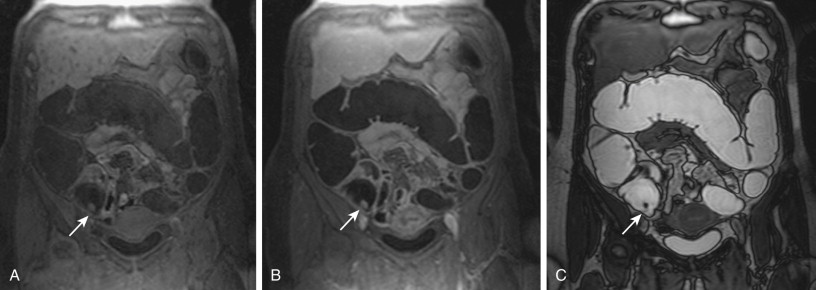
Whenever a colorectal lesion is found, the analysis has to be repeated using the corresponding, native, T1-weighted dataset. The signal intensities of the lesions should be recorded to calculate the percentage of contrast enhancement. This procedure helps distinguish between residual stool and true colorectal polyps or cancers reliably. Although true colorectal lesions always enhance ( Figs. 54-1 and 54-2 ), residual stool shows the same signal properties on native and contrast-enhanced T1-weighted images. However, scar tissue, (e.g., after appendectomy) may also show a significant percentage of contrast enhancement, thereby mimicking a polyp ( Fig. 54-3 ). In a second step, the FISP sequences should be analyzed. These sequences have been found to be very accurate for the detection of inflammatory bowel lesions.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree