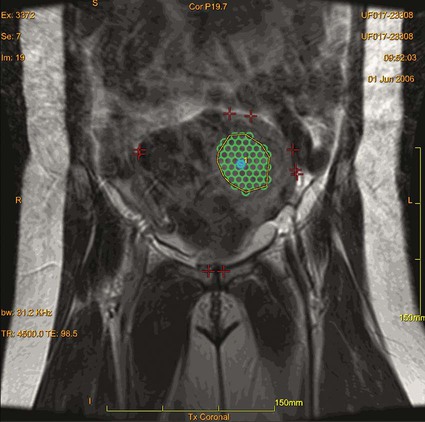
Uterine leiomyomas, also called fibroids, are gonadal steroid-dependent benign smooth-muscle tumors arising from uterine muscle tissue. Fibroids are estimated to occur in 20% to 50% of women of reproductive age,1 representing the most common female pelvic tumor. The incidence of uterine fibroids is two to three times higher in African American women, who also present at a younger age and with more symptomatic cases.2 Treatment of fibroids is reserved for symptomatic myomas and those thought to contribute to difficulties with fertility. Significant symptoms related to these tumors occur in up to 25% of women and include menorrhagia and pressure-related symptoms such as pelvic fullness, urinary urgency and frequency, and dyspareunia. It is thought that a reduction of fibroid size strongly correlates with a reduction in pelvic symptoms.3 Laparoscopic and hysteroscopic myomectomies are minimally invasive options performed when the patient wants to preserve fertility through selective resection of the fibroids while leaving the uterus behind. Unfortunately, these treatment options can only be performed in selected cases, depending on size and location of the myomas.4 Robot-assisted laparoscopic myomectomy represents the most recent surgical advance in uterine-sparing surgery, but safe application of this technique is also limited, depending on the number and size of the fibroids and condition of the uterus.5 Uterine fibroid embolization is another alternative that is good for treating a wide range of fibroid locations and sizes. However, the procedure leads to amenorrhea in 1% to 2% of women younger than 45 and 15% of women older than 45, and may be associated with postembolization syndrome (postprocedural pain and fatigue), which results on average in an 8- to 10-day recovery.6 Ultrasound-induced thermal coagulative necrosis is a thermoablative technique that has been studied for over 60 years and was first used in 1942.7 However, clinical applications were hampered by the inability to accurately target the focus and control temperature during the procedure. Recent advances in MRI have overcome these limitations. In the case of fibroids, MRI has excellent anatomic resolution for targeting, high sensitivity for localizing tumors, and enables real-time monitoring of temperature changes induced by ultrasound sonications during the procedure in the targeted tissues.8 This last feature provides necessary feedback for intraprocedural monitoring of MRgFUS therapy. Focused ultrasound has been applied in the treatment of a variety of clinical conditions, including prostate hyperplasia and cancer, kidney tumors, and breast lesions.9 In October 2004, MRgFUS for fibroid treatment received approval by the U.S. Food and Drug Administration (FDA), representing the first commercially available method of treating patients with focused ultrasound in the United States (ExAblate 2000 [InSightec, Haifa, Israel]). Patients with other pelvic diseases (i.e., endometriosis or dermoids) may not be suitable for MRgFUS. Other exclusions include pregnancy, calcified fibroids, and surgical clips or intrauterine devices (IUDs), which could reflect focused ultrasound energy to other locations. Women who are not candidates for MRI, such as those with cardiac pacemakers, should not receive this treatment. Several recent articles on MRgFUS of adenomyosis have demonstrated promise for this modality in treating this entity, initially felt to be a relative contraindication.10 Previous experience has shown that abdominal scars have higher ultrasound absorption compared to regular tissue and when in the ultrasound beam path may lead to pain and thermal skin damage; therefore, patients with cutaneous scars in the proposed beam path may have to be excluded.11 The fibroid(s) to be treated must be accessible, and its center can be no more than 12 cm from the skin surface, which is no more than 20 cm from the focused ultrasound transducer using existing technology. Homogeneously dark fibroids on T2-weighted spin-echo MRI respond well to MRgFUS. Fibroid tissue that is bright on T2-weighted spin-echo sequences has been thought by several investigators to respond less well to standard sonications and has previously been considered a relative contraindication. However, a recent publication showed no statistically significant correlation between T2 appearance and 12-month clinical and imaging outcome so long as sufficiently high energies are used to treat these fibroids.12 The first FDA-approved clinical MRgFUS system (ExAblate 2000) was developed by InSightec Inc. (Haifa, Israel). It is built into a modified MRI table that docks with a compatible 1.5T or 3T GE MR scanner.13 The ultrasound transducer is located in a water tank within the ExAblate table and covered by a thin plastic membrane, which allows the ultrasound beam to propagate through to the patient from the table.7 Some anatomic issues must be considered. When performing the treatment, it is of great importance to check the far field of the ultrasound beam. In the series by Stewart et al.,14 the most serious complication after MRgFUS was development of sciatic nerve palsy, which resolved by the 12-month follow-up visit. In this case, the fibroid was posteriorly located and, reviewing all treatment images, the nerve was not directly sonicated, but heat transfer from the adjacent pelvic bones led to indirect injury of the nerve. Furthermore, it is also essential to check the proximal field before treatment, because critical structures interposed in the treatment field may be harmed. Air-filled viscera such as the small intestine can interfere in the treatment, reflecting the ultrasound beam, injuring the intestine, and reducing the overall effectiveness. Most physicians are familiar with diagnostic ultrasound, which operates at varied frequencies ranging from 1 to 20 MHz. On the other hand, frequencies of 0.8 to 3.5 MHz are generally employed during clinical applications of focused ultrasound, and the energy delivered to the focal spot during focused ultrasound sessions is several times greater than diagnostic ultrasound beams.15 Similar to focusing light, the ultrasound waves can be brought to a tight focus, delivering an intense amount of energy to a specific volume while sparing surrounding tissues. This focusing is generally done by using a specially designed concave ultrasound transducer or, alternatively, by using a lens in front of a more standard-shaped ultrasound transducer. Variations on the focal spot location can be achieved using a phased-array transducer. Ultrasound causes tissue damage through two primary mechanisms: (1) conversion of mechanical energy into heat and (2) cavitation.16 It is important to consider the local tissue environment and content through which the beam passes because they have an important effect on the ultimate achieved temperature at the desired focus. Heterogeneous tissue can cause sound-wave reflections that interrupt the beam or divert the focused energy to a slightly different location than theoretically predicted. Focused ultrasound can rapidly elevate the temperature at the focus to above 80°C, leading to cell death in less than a second of exposure. The volume of ablation after a single focused ultrasound sonication depends on the transducer and is generally small and usually cigar-shaped, measuring 1 to 3 mm × 8 to 15 mm.15 Therefore, several adjacent focused ultrasound sonications are required to ablate a larger volume (Fig. e149-1). Because cavitation is less predictable, it is usually less desired. Cavitation is based on the principle that ultrasound waves subject the tissue molecules to alternating cycles of compression and rarefaction, during which gas can be drawn out of solution, creating bubbles. These bubbles oscillate in size and ultimately might collapse, causing local energy release.15 Thus, the final tissue lesion is generated by a combination of mechanical stress and heat. Because heterogeneity of the tissues may cause deviations of the focused ultrasound beam from the theoretical expected path, it is important to use a feedback mechanism to ensure accurate tissue targeting. Noninvasive magnetic resonance thermometry (MRT) provides such a feedback mechanism.17 Noninvasive multiplanar temperature maps are feasible with MRI and may be based on the T1 relaxation time, the diffusion coefficient, or proton resonance frequency (PRF) of tissue water.17 The PRF method is the most frequently employed technique and uses fast spoiled gradient-recalled echo sequences. The MR images during the ultrasound sonication are compared with images obtained immediately before that sonication. The changes in those images can then be calibrated to generate real-time feedback temperature mapping during the entire procedure, ensuring the thermal ablation is located in the previously determined target while avoiding damage to surrounding critical tissues.18 By initially using a lower level of MRgFUS energy, one can confirm correct targeting by detecting the location of a small temperature elevation before providing the full energy level that would cause irreversible damage. This type of safety mechanism is employed in clinical MR-guided MRgFUS systems.
Magnetic Resonance–Guided Focused Ultrasound Treatment of Uterine Leiomyomas
Indications
Contraindications
Equipment
Technique
Anatomy and Approaches
Technical Aspects
Principles of Focused Ultrasound Imaging
Principles of Magnetic Resonance Thermometry
Magnetic Resonance–Guided Focused Ultrasound Treatment of Uterine Leiomyomas