The optimal strategy for prostate cancer diagnosis is to avoid overdiagnosis, defined as diagnosis of clinically insignificant disease, and undersampling of the gland, which leads to missing clinically significant disease. Targeted prostate biopsy is a potential solution for decreasing the rate of both overdiagnosis and undersampling of prostate cancer. We focus here on different techniques for targeting prostate lesions identified on multiparametric MR imaging and review different clinical settings in which MR imaging–targeted prostate biopsies are performed.
Key points
- •
Magnetic resonance (MR)-targeted prostate biopsy is beneficial in most scenarios of prostate cancer diagnosis and management.
- •
Targeted biopsies can detect more clinically significant disease requiring curative treatment, and avoid detection of insignificant disease that is commonly diagnosed on random systematic biopsy.
- •
Fewer biopsy cores are needed when targeted prostate biopsy is used and can reduce complication rate.
- •
MR-targeted biopsy limitations are a potential for missing small volume prostate cancer on multiparametric MR imaging, equipment complexity and availability, and operator training requirement.
Introduction
The optimal strategy for prostate cancer (PCa) diagnosis is to avoid overdiagnosis, defined as diagnosis of clinically insignificant disease, and undersampling of the gland, which leads to missing clinically significant disease (CSD). Targeted prostate biopsy is a potential solution for decreasing the rate of both overdiagnosis and undersampling PCa.
Recent applications of multiparametric prostate MR imaging (MP-MR imaging) have been documented as aiding more accurate risk stratification of PCa and treatment selection. MR-invisible PCa has been shown to be associated with lower risk of adverse biopsy pathology.
The management of patients with an increased prostate-specific antigen (PSA) level and negative standard transrectal ultrasound (TRUS)-guided prostate biopsy results is a challenging clinical setting in which MP-MR imaging may detect cancer lesions missed on standard TRUS-guided biopsy. The anterior prostate is the primary site of missed tumors and these can be detected on MP-MR imaging. It has been suggested that MP-MR imaging could be used as a triage test for the management of patients with increasing PSA levels and negative prior biopsy.
In this article, we focus on different techniques for targeting prostate lesions identified on MP-MR imaging and review different clinical settings in which MR imaging–targeted prostate biopsies are performed.
Introduction
The optimal strategy for prostate cancer (PCa) diagnosis is to avoid overdiagnosis, defined as diagnosis of clinically insignificant disease, and undersampling of the gland, which leads to missing clinically significant disease (CSD). Targeted prostate biopsy is a potential solution for decreasing the rate of both overdiagnosis and undersampling PCa.
Recent applications of multiparametric prostate MR imaging (MP-MR imaging) have been documented as aiding more accurate risk stratification of PCa and treatment selection. MR-invisible PCa has been shown to be associated with lower risk of adverse biopsy pathology.
The management of patients with an increased prostate-specific antigen (PSA) level and negative standard transrectal ultrasound (TRUS)-guided prostate biopsy results is a challenging clinical setting in which MP-MR imaging may detect cancer lesions missed on standard TRUS-guided biopsy. The anterior prostate is the primary site of missed tumors and these can be detected on MP-MR imaging. It has been suggested that MP-MR imaging could be used as a triage test for the management of patients with increasing PSA levels and negative prior biopsy.
In this article, we focus on different techniques for targeting prostate lesions identified on MP-MR imaging and review different clinical settings in which MR imaging–targeted prostate biopsies are performed.
Utility of multiparametric MR imaging
With the MP-MR imaging approach to imaging of the prostate, the use of functional MR imaging sequences such as diffusion-weighted imaging and dynamic-contrast enhanced imaging in addition to anatomic assessment offers an important advantage in the diagnosis and localization of PCa. It has been reported that MP-MR imaging may decrease the number of unnecessary repeat biopsies in approximately 31% of men with initial negative extended transperineal biopsy. The sensitivity and specificity of MP-MR imaging in the overall diagnosis of PCa versus diagnosis of CSD was reported as 82.2% versus 100% and 77.8% versus 78.9%, respectively.
Bratan and colleagues showed that 3T MP-MR imaging could detect 93% of high-grade PCa (Gleason score of >7) with any volume, and 95.8% of those greater than 0.5 mL in reference to radical prostatectomy pathology findings. The sensitivity to diagnose PCa with unfavorable grade (Gleason score of ≥7) was 88.1%. The sensitivity for diagnosing low-grade (Gleason score of 6) and large volume disease (>0.5 mL) was 69.6%.
Risk stratification is the other potential application of MP-MR imaging. In a study by Marcus and colleagues, the highest yield of MP-MR imaging for risk stratification with impact on disease management was demonstrated in intermediate risk disease in which MR imaging led to upstaging in 25.6% of cases. MR level of suspicion for PCa was shown to be correlated significantly with D’Amico risk stratification.
Although MP-MR imaging is an adjunct tool for PCa diagnosis and management, its role is being evaluated in different patient workup algorithms. MR-detected suspicious lesions of the prostate can be targeted for tissue sampling using a variety of image-guided biopsy techniques in many PCa management scenarios.
Targeted biopsy techniques
Targeting suspicious lesions in the prostate can be achieved directly within the MR imaging scanner, “in-bore” MR-guided biopsy (MR-GB), or outside the MR imaging using fusion techniques that allow utilization of preprocedure MP-MR imaging and application of TRUS for imaging guidance during the procedure. Two image fusion techniques can be used: (1) a “cognitive” fusion by an operator performing the US-guided procedure who is familiar with MR imaging results and TRUS, and (2) software-based coregistration of MR images to TRUS images (MR-TRUS fusion) enabling the real-time TRUS-guided biopsy.
Known challenges to MR-guided “in-bore” biopsies include limited access within the scanner for manual instrument handling. A solution to this problem is the use of MR-compatible robots designed to operate in the space and environmental restrictions inside the MR scanner. Application of MR imaging compatible robotic devices involves sophisticated engineering solutions because, in addition to restrictions in materials that can be used to build these tools, robots require actuators and sensors that can only use a certain type of energies, and dedicated software applications are needed for image to robot registration. A number of MR-compatible robots, ranging from simple manipulator to fully automated systems, have been developed over the years. The robotic approach to prostate biopsy offers a possibility of more precise targeting that may be crucial to the success of prostate interventions.
There are several platforms currently available for MR-TRUS fusion biopsies. The advantages on these fusion techniques include performance in the standard clinical setting familiar to the patient and the operator, and shorter procedure times. The disadvantages may be the lower targeting accuracy for small lesions and complicated fusion software handling.
In-Bore Magnetic Resonance-Guided Biopsy
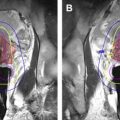
In the “in-bore” technique, lesions identified as indeterminate or suspicious on diagnostic MP-MR imaging are being targeted while the patient is in the MR imaging scanner ( Figs. 1 and 2 ). Both transrectal and transperineal approaches have been reported using this technique. Prebiopsy MP-MR imaging is performed to identify target lesions. Patients are placed in the prone, supine, or decubitus position. Body surface coils are used to improve the signal to noise ratio. Typically, an MR biopsy guide is inserted into the rectum (ie, contrast-filled endorectal needle guide) and multiplanar T2-weighted fast spin echo images are acquired for target localization. The position of the MR biopsy guide is adjusted manually based on the location of the target and automated software calculations, and then the biopsy needle is advanced through the needle guide toward the suspicious lesion. The needle position in relation to the lesion is imaged for confirmation using MR sequences such as T2-weighted fast spin echo, single shot fast spin echo, or TRUE-fast spin echo images. Once the needle position is aligned with the target, the needle is advanced and the biopsy performed. Needle localization may be reconfirmed and tracked by further scanning after tissue sampling. When the robotic device is used for MR-GB, it is fixed typically to the scanner table and the needle guide is inserted into the rectum or perineum. After registration of the robot and the needle guide with localization sequences, the robot is controlled remotely to achieve the desired angulation for inserting the needle to target the suspicious lesion.
Researchers from the National Cancer Institute assessed the accuracy of needle placement and sampling on transrectal MR-GB and reported that 28% of the biopsies had an error of greater than 5 mm, one that may lead to missing CSD defined as a tumor volume more than 0.5 cm 3 . Further investigations are needed to improve targeting and develop methods to compensate for prostate gland displacements.
Transperineal MR-guided prostate biopsy was introduced initially by researchers from the Brigham and Women’s Hospital. Recently, they reported the performance of robotically assisted needle guide placement using transperineal MR-GB and compared that method with the manual placement through the template guide. The robotic device used in this study applied a motorized template to guide the needle with a control resolution of 0.001 mm. The investigators used 3DSlicer (this is the name of open source software http://www.slicer.org/ ) software for motion control of the robotic device and to optimize the needle placement. T2-weighted images were obtained after each motion of the robotic device to reconfirm the location of the needle. The robotic approach was more accurate than the manual approach in terms of best needle placement attempt. Both mean time per core procedure and whole procedure time were shorter with the robotic method. These robotically assisted techniques require a complex setting, a long time to perform, and specific equipment that is not widely available; in addition, they are costly.
Although MR-GB offers accurate targeting of prostate lesions, these procedures may be subject to the sampling error, inaccurate needle placement secondary to prostate deformation and displacement, and patient movement owing to discomfort. Because no real-time tracking of the prostate location is available during MR-GB, the procedure is lengthened by frequent confirmatory scans that need to be performed to adjust the needle and target alignment.
Magnetic Resonance–Ultrasound Fusion Biopsy
Cognitive registration
Cognitive fusion allows the physician performing the prostate biopsy to use the information from MP-MR imaging that is reviewed before the procedure. Suspicious lesions identified on the MR imaging are targeted with the usual TRUS equipment based on the anatomic location of the lesion. A prospective, multicenter study demonstrated the advantage of cognitive MR-TRUS fusion biopsy compared with systematic TRUS biopsy. A biopsy with cognitive targeting led to a 10% increase in cancer detection and 15% increase in high-grade disease detection.
Software-based registration
To overcome the limitations of cognitive fusion, different software-based image fusion techniques have been developed to assist in registration of real time TRUS images with preprocedure MP-MR imaging, to decrease the procedure time and complexity of equipment, and to allow performance of the targeted biopsy in a standard clinical office.
MP-MR images acquired before the biopsy procedure are analyzed using the postprocessing software, and 3D segmentation of the prostate gland is performed. Additionally, at the time of TRUS, 2-dimensional (2D) images that are acquired are processed by the software and reconstructed into a 3D model. Subsequently, navigational software overlays or fuses MR images to the real-time TRUS images (GE Logiq E9, GE Healthcare, Milwaukee, WI; Fig. 3 ) or performs 3D MR imaging to 3D TRUS datasets registration (UroNav, Philips-Invivo, Gainesville, FL; Fig. 4 ), enabling the operator to target the suspicious lesion on MR imaging as evident on fused images.