Uterine leiomyomas are the most common female pelvic neoplasm. Commonly referred to as “fibroids,” they consist of smooth muscle tissue and a fibrous extracellular matrix. They can be solitary but are often multiple, varying in size and location throughout the uterus. The prevalence of fibroids increases toward the end of a woman’s reproductive cycle and decreases after menopause. The incidence of uterine fibroids varies widely, depending on the method of diagnosis, and they are more common in the African American population.
Benign clonal tumors of smooth muscle cells, the etiology and pathogenesis of uterine fibroids remain unknown. They are steroid-dependent tumors that respond to treatment with gonadotropin-releasing hormone (GnRH) agonists, and roles for both estrogen and progesterone have been found in their growth. Although many women with uterine fibroids are asymptomatic, many others suffer from symptoms such as pelvic pain, menorrhagia, urinary frequency, back pain, dysmenorrhagia, dyspareunia, and infertility. Fibroids are usually classified according to their location (subserosal, intramural, submucosal), which in turn influences potential treatment options. Surgical treatments range from hysterectomy to myomectomy, and the approach to the latter depends on the location of the fibroid. Subserosal fibroids are on the external surface of the uterus and as such may be amenable to laparoscopic removal; a submucosal fibroid may be seen to distort the uterine cavity, and may be accessible to transvaginal hysteroscopic resection.
Diagnosis
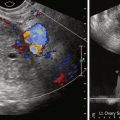
Pelvic or transvaginal ultrasonography is the first imaging modality of choice, usually demonstrating a well-defined hypoechoic mass. Fibroids typically appear as solid areas with refractive shadows. Ultrasound can be limited by patient body habitus, however, and it can be difficult to evaluate large fibroids that extend out of the pelvis. The imaging features of uterine fibroids are discussed in more detail in Chapter 15 . For accurate fibroid detection, localization, and volumetric analysis, magnetic resonance imaging (MRI) is the modality of choice because of its inherent soft tissue contrast resolution and multiplanar imaging capability. MRI is also an excellent tool in helping differentiate fibroids from other uterine pathologies, such as adenomyosis, which may present with similar symptomatology.
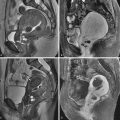
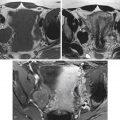
As outlined in Table 38-1 , fibroids demonstrate variable imaging characteristics on MRI. Typically fibroids appear as a well-defined mass, are of low signal intensity (SI) on T2-weighted images ( Figure 38-1 ) and enhance after administration of an intravenous contrast agent. A less common hypercellular group of fibroids are of high SI on T2-weighted images ( Figure 38-2 ), thought to represent hypercellular tumors composed of smooth muscle cells with few or no intervening collagen fibers. As they increase in size, fibroids may undergo hyaline degeneration. Hyaline degeneration usually appears as low signal on T2-weighted images (even lower than in usual fibroids, but the difference can be difficult to discern), although a heterogeneous pattern of distinct low signal intensity with a speckled appearance has been reported ( Figure 38-3 ). Hemorrhagic degeneration may be best seen as areas of increased SI on T1-weighted images ( Figure 38-4 ), with lack on enhancement after administration of intravenous contrast. Areas of calcification may form, usually along the periphery of the fibroid, which are best detected on gradient echo T1-weighted images. A rare variation of uterine fibroid is the lipoleiomyoma, which consists of various amounts of smooth muscle cells, mature adipose tissue, and fibrous tissue ( Figure 38-5 ).
| Underlying Pathogenesis | T2 Signal intensity | T1 Signal intensity | Contrast-Enhanced T1 |
|---|---|---|---|
| Hypointense | Isointense to muscle | Enhances similar to muscle |
| Hyperintense | Isointense to muscle | Enhances similar to muscle |
| Usually hypointense, occasionally heterogeneous | Usually isointense to muscle | Heterogeneous enhancement |
|
| May be hyperintense/isointense (depending on age of blood) | No enhancement |
| Hyperintense with hypointense amorphous areas | Hyperintense (which suppress on fat-suppressed images) with hypointense amorphous areas | Enhancement of amorphous areas |






Treatment Options
Current treatment options for uterine fibroids range from expectant management to medical management to uterine artery embolization to myomectomy and hysterectomy. Medical management usually involves hormonal manipulation with GnRH agonists, which decrease blood flow to the fibroid and cause a temporary reduction in fibroid size. This medical menopause may also be induced through use of GnRH antagonists, which have a more rapid onset of effect, but long-term usage of either the agonists or antagonists is limited because of the menopausal side effects involved.
As recently as 15 years ago, the choices for a woman with symptomatic fibroids were confined to conventional hysterectomy and conventional abdominal myomectomy. Hysterectomy has always been considered the standard of care for symptomatic uterine fibroids, being the leading reason for hysterectomy in the United States. However, although this is a definitive procedure that will eliminate symptoms permanently, it is associated with surgical complication risks. Women face lengthy recovery times, absenteeism, and potentially negative quality-of-life outcomes associated with uterine loss. A recent study that evaluated surgical treatment options for uterine fibroids found that although surgical treatment costs were high, absenteeism and disability were also important components of the cost burden of surgical fibroid treatment for women, their employers, and the health care system. Hysterectomy is also not appropriate for women who wish to preserve their fertility. Myomectomy is often considered the treatment of choice for women who express a desire for future childbearing, but it may not be completely effective in eliminating fibroid symptoms because the fibroid may not be removed in its entirety. Myomectomy is associated with surgical risks, as well as a risk for uterine rupture and complications in any subsequent pregnancy.
Perhaps motivated by a trend toward later childbearing and fertility preservation, as well as the need for reduced recovery time, many women are increasingly seeking less invasive treatment options. An option that has seen significant growth and interest since its first introduction in 1995 is uterine artery embolization (UAE), which is discussed in more detail in Chapter 39 . This involves the selective embolization via catheter delivery of embolic material of uterine artery branches that perfuse the fibroids. UAE has a lower rate of major complications than hysterectomy or myomectomy, but a higher rate of minor complications, and is associated with more urgent visits and rehospitalizations. Two recent large European studies, the multicenter randomized EMMY (EMbolization vs. hysterectoMY) trial and the HOPEFUL study, found that from a societal economic perspective, UAE is the superior treatment strategy to hysterectomy in women with symptomatic uterine fibroids, although the need to improve the management of expectations after UAE, particularly regarding fertility, was emphasized.
Magnetic resonance-guided focused ultrasound surgery (MRgFUS) is a novel noninvasive outpatient thermoablative treatment option, first introduced in the United States by the team at Brigham and Women’s Hospital (Boston, MA). It was approved by the Food and Drug Administration (FDA) in 2004 for fibroid treatment. The system includes an MRI system integrated with an ultrasound thermal ablation device in the table that is used to ablate tissue. MRgFUS is currently being examined for use in treatment of benign and malignant tumors throughout the body. Much of the worldwide experience with MRgFUS has been with treatment of uterine fibroids, however, with more than 5000 treatments to date. MRgFUS offers several potential advantages over conventional treatments, such as hysterectomy, myomectomy, and even UAE. It is a completely noninvasive outpatient procedure that usually takes approximately 3 hours and is performed under minimal conscious sedation. Patients usually return to their normal activities within 24 hours compared with 10 days with UAE and 6 weeks with hysterectomy. It has a low risk for postprocedure complications because the ablated tissue is absorbed by the body, decreasing the risk for postembolization syndrome often associated with UAE.
Magnetic Resonance-Guided Focused Ultrasound Surgery
Fundamental Principles
As ultrasound waves interact with tissue and lose energy through tissue absorption, a localized increase in temperature within the tissue results. If the temperature elevation is large enough, and maintained for long enough, this thermal effect can cause tissue coagulation or ablation. Just as the concept of embolization was not new when it was first reported in the treatment of fibroids, focused ultrasound surgery (FUS) was also by no means new when first used in fibroid treatments. The first therapeutic use of FUS in humans was performed by Lynn et al. in 1942. In the 1950s the Fry brothers used a complex FUS system for localizing targets within the brain. More recently, ultrasound guidance of high intensity focused ultrasound has been made possible for targeting of soft tissue, and is used extensively in many centers. Clinical acceptance of FUS treatment worldwide, particularly in the United States, was hampered for many years because of poor definition and guidance of the target beam, difficulty in controlling the ablation spot position, and lack of real-time feedback of thermal damage.
MRI monitoring of the thermal ablation, first described in 1988, has led to the advent of MRgFUS, wherein MRI offers anatomic, functional, and thermal guidance during delivery of the FUS beam. The excellent anatomic resolution offers accurate lesion visualization and treatment planning. It also offers continuous imaging of the fibroid and adjacent structures such as bowel, sacral nerves, and bladder during treatment. By exploiting the temperature sensitivity of the water proton chemical shift, MR thermometry is made possible. The physical basis for the temperature dependence of the proton resonance frequency shift is that an increase in temperature leads to a corresponding increase in brownian motion of the water molecules and a breakdown in hydrogen bonding, which results in a linear shift in the water proton resonance frequency. Phase imaging is used to estimate the proton resonance frequency shift using a fast spoiled two-dimensional gradient-recalled echo sequence before, during, and after each sonication to monitor tissue temperature elevations within the targeted tissue to be treated ( Figure 38-6 ), and also in the surrounding normal tissue to monitor for inadvertent thermal deposition.

Magnetic Resonance-Guided Focused Ultrasound System
The ExAblate 2000 (Insightec Inc, Haifa, Israel) is the first commercial, and currently the only FDA-approved, MRgFUS device for treatment of uterine fibroids. Developed in collaboration with investigators at Brigham and Women’s Hospital, this system uses a multielement phased array transducer. The energy originates from multiple electronically controlled transducer elements (phased arrays) arising from a spherically curved ultrasound transducer ( Figure 38-7 ). The transducer has 208 elements, a 120-mm diameter, and a 160-mm radius of curvature. The frequency of the ultrasound beam generated ranges from 0.9 to 1.3 MHz. The system can focus to a point deep within the body, with each element driven by radiofrequency signals of a specified phase and amplitude so that waves emitted are all in phase at the focal point, and subsequently at the point of convergence there is substantial temperature increase.