MR imaging plays a key role in staging evaluation of rectal cancer. The cornerstone of staging MR involves high-resolution T2 imaging orthogonal to the rectal lumen. The goals of MR staging are identification of patients who will benefit from neoadjuvant therapy prior to surgery to minimize postoperative recurrence and planning of optimal surgical approach. MR provides excellent anatomic visualization of the rectum and mesorectal fascia, allowing for accurate prediction of circumferential resection margin status and tumor stage. MR has an evolving role for the evaluation of neoadjuvant treatment response, further triaging optimal patient treatment and surgical approach.
Key points
- •
Magnetic resonance (MR) imaging plays a key role in staging evaluation of rectal cancer. The cornerstone of staging MR involves high-resolution T2 imaging orthogonal to the rectal lumen.
- •
The goals of MR staging are identification of patients who will benefit from neoadjuvant therapy prior to surgery in order to minimize postoperative recurrence and planning of optimal surgical approach.
- •
MR provides excellent anatomic visualization of the rectum and mesorectal fascia (MRF), allowing for accurate prediction of circumferential resection margin (CRM) status and tumor stage.
- •
Improved accuracy for lymph node staging can be achieved through evaluation of node morphology, signal heterogeneity, and dynamic enhancement characteristics in addition to size.
- •
MR has an evolving role for the evaluation of neoadjuvant treatment response, further triaging optimal patient treatment and surgical approach.
Introduction
Rectal cancer is one of the most common malignancies, with an incidence of 40 in 100,000. Colorectal cancer is the third leading cause of cancer-related mortality worldwide in both men and women. In 2013, an estimated 140,000 new colorectal malignancies will be diagnosed in the United States alone, and more than one-quarter of these will arise from the rectum. Rectal cancers are associated with poorer prognosis and higher local recurrence than their counterparts in the colon. Although precise staging of rectal cancer is possible only with a surgical specimen, progress in preoperative management has necessitated the advent of accurate staging methods prior to surgical resection. Total mesorectal excision (TME) is the standard of care, with additional neoadjuvant concurrent chemoradiation therapy (CCRT) in a select group of patients. In patients with advanced local-stage disease, neoadjuvant CCRT has improved local control and is associated with reduced toxicity when compared with postoperative adjuvant therapy. As such, initial staging investigations should identify the patients who benefit from preoperative therapy with intent to minimize the risk of tumor recurrence, while avoiding unnecessary treatment of patients in whom no benefit has been shown. Preoperative staging can also assist in surgical planning, identifying candidates for sphincter-preserving surgery. In this regard, high spatial resolution MR imaging has been established as an accurate preoperative staging technique and plays a critical role in pretreatment staging and evaluation for recurrence. In more recent years, the role of MR imaging has expanded to include evaluation of neoadjuvant therapy response, further tailoring surgical approaches and oncologic treatment options. MR restaging is of particular utility at institutions that have adopted organ-preserving techniques, such as transanal excision and nonoperative management (ie, a wait-and-see approach) for clinical CR.
Introduction
Rectal cancer is one of the most common malignancies, with an incidence of 40 in 100,000. Colorectal cancer is the third leading cause of cancer-related mortality worldwide in both men and women. In 2013, an estimated 140,000 new colorectal malignancies will be diagnosed in the United States alone, and more than one-quarter of these will arise from the rectum. Rectal cancers are associated with poorer prognosis and higher local recurrence than their counterparts in the colon. Although precise staging of rectal cancer is possible only with a surgical specimen, progress in preoperative management has necessitated the advent of accurate staging methods prior to surgical resection. Total mesorectal excision (TME) is the standard of care, with additional neoadjuvant concurrent chemoradiation therapy (CCRT) in a select group of patients. In patients with advanced local-stage disease, neoadjuvant CCRT has improved local control and is associated with reduced toxicity when compared with postoperative adjuvant therapy. As such, initial staging investigations should identify the patients who benefit from preoperative therapy with intent to minimize the risk of tumor recurrence, while avoiding unnecessary treatment of patients in whom no benefit has been shown. Preoperative staging can also assist in surgical planning, identifying candidates for sphincter-preserving surgery. In this regard, high spatial resolution MR imaging has been established as an accurate preoperative staging technique and plays a critical role in pretreatment staging and evaluation for recurrence. In more recent years, the role of MR imaging has expanded to include evaluation of neoadjuvant therapy response, further tailoring surgical approaches and oncologic treatment options. MR restaging is of particular utility at institutions that have adopted organ-preserving techniques, such as transanal excision and nonoperative management (ie, a wait-and-see approach) for clinical CR.
Current concepts in rectal cancer
Surgical resection is the mainstay of curative therapy for rectal cancer. TME is the standard of care, which involves radical removal of the entire rectal compartment with surrounding mesorectum through identification of naturally occurring tissue planes of the MRF. The risk of local recurrence is considerably lower when the rectum is excised intact. For tumors in the high rectum, low anterior resection (LAR) is the surgery of choice, whereby a portion of the distal rectum can be preserved. Tumors in the mid- to low-rectum are resected to the level of the pelvic floor muscles. For more distal tumors, sphincter-sparing surgeries, such as ultra-LAR with coloanal anastomosis and intersphincteric resection with coloanal anastomosis, may be attempted. Utilization of a sphincter-sparing technique results in improved surgical outcome in terms of recurrence and postoperative leak and substantially improves quality of life in postoperative patients. Abdominoperineal resection (APR) includes removal of the entire sphincter complex and is reserved for tumors in the low rectum that are fixed to adjacent pelvic organs or structures. Patients with operable locally advanced tumor or recurrent tumors are considered for total pelvic exenteration, which involves complete resection of the pelvic viscera and draining lymphatics, with the objective of removing all malignant disease.
The CRM is a pathologic term that refers to the surgically dissected surface of the specimen and corresponds to the nonperitonealized portion of the rectum. A negative CRM is defined as greater than or equal to 1 mm between the tumor edge and the surgical margin and is associated with a significantly lower risk of local recurrence than a positive CRM (<1 mm). The relationship of tumor to the anterior peritoneal reflection is important when considering CRM, because the anterior peritoneal reflection extends in a nearly circumferential fashion around the upper rectum but only involves the anterior aspect of the lower rectum ( Fig. 1 ). The term, CRM, is not appropriate for tumors involving the upper and/or anterior peritonealized portion of the rectum.
The MRF is the extraperitoneal pelvic fascial plane that surrounds the mesorectum. CRM is defined by the surgical specimen, the goal of which is to approximate MRF. MRF is visualized on MR imaging and is a more appropriate term for MR reporting. As with CRM, the term MRF only applies to nonperitonealized portion of the rectum.
Imaging modalities in rectal cancer diagnosis and staging
MR imaging is the primary imaging modality for local staging evaluation for rectal cancer. Compared with other cross-sectional imaging modalities, the main benefits of MR imaging include high soft tissue contrast resolution for tumor delineation and ability to visualize and assess MRF/CRM and its relationship to the tumor and evaluation of regional lymph nodes. MR has an established role in initial staging but in more recent years utilization has evolved to include evaluation of treatment response and local recurrence.
Endorectal ultrasound (EUS) is an accurate method for evaluating involvement of mural invasion in early-stage rectal adenocarcinoma and in some institutions is used in staging of rectal cancer. Although EUS is accurate for staging superficial rectal tumors, it has limited utility in the staging of more advanced disease, because the depth of acoustic penetration is generally unable to assess advanced-stage disease. In a prospective study by Fernandez-Esparrach and colleagues, T staging accuracy for EUS was similar to MR imaging for T2 and T3 tumors. MR imaging did not visualize any T1 tumor, and EUS understaged all T4 tumors. Accuracy of MR for nodal staging is higher than EUS. EUS also tends to overstage T2 tumors. In a randomized controlled trial by Sauer and colleagues, 20% of tumors staged as T3 or T4 by EUS were pathologically staged as T2. Additional limitations include operator dependence, patient tolerance, probe limitations, and inability to examine proximal to obstructing tumors. Hence, EUS has a high accuracy in the evaluation of early-stage tumors but is less suitable than MR imaging for the evaluation of the mesorectal excision plane or evaluation of mesorectal and extramesorectal lymph nodes.
CT is not recommended for local staging of rectal cancer, because it cannot reliably differentiate layers of the rectal wall, identify the MRF, or depict tumor invasion in surrounding pelvic structures. In evaluation of the MRF, multidetector-row CT does not correlate well enough with MR imaging findings to replace it in rectal cancer staging. CT is essentially used to assess distant metastatic disease, such as liver and lung metastases.
MR imaging technique
MR technique is somewhat variable according to institution, but the universal goal of MR imaging is acquisition of high-resolution T2-weighted images with small field of view (FOV) thin-section imaging for evaluation of the primary tumor and its relationship to pelvic structures as well as assessment regional lymph nodes.
Prior to an MR imaging examination, obtaining clinical information assists in planning the MR scan, including tumor location, circumferential extent, and distance from anal verge. The examination may take up 45 minutes, so patients should be positioned comfortably in a supine position. In general, no bowel preparation is required. An empty bladder is preferable. Use of an antispasmodic agent (ie, glucagon or buscopan) helps eliminate bowel motion artifact and is used at the authors’ institution in the absence of contraindications. In patients who cannot tolerate the length of an examination, obtaining a high-resolution T2 sequence is critical, and the protocol may be modified to accomplish this by shortening or omission of other T2 sequences. Use of gel or luminal contrast in staging of primary rectal cancer is controversial. Distending the rectum with gel may alter the distance from MRF or the sphincter complex, and instillation of gel may stimulate rectal sphincter contraction, potentially resulting in motion artifact. Some studies suggest benefit, however, in staging of smaller or polypoid tumors or in patients who have had prior radiation. Gel can also help identify the location of attachment to the rectal wall. If rectal gel or luminal contrast is considered, no more than 60 to 100 cm 3 is recommended. In the authors’ practice, rectal MR imaging is performed without utilization of rectal gel or luminal contrast.
Phased-array body/pelvis coils provide better signal, greater coverage, and more homogeneity than endorectal (surface) coils. The coil should be positioned to cover rectum, mesorectum, and highest nodal drainage bin, approximately 5 cm superior to the tumor. In general, this provides coverage from sacral promontory to below pubic symphysis. The lower edge of coil should be 10 cm below the pubic symphysis to acquire adequate signal from the low rectum and anus. A 3.0-T system can provide faster image acquisition and improved signal-to-noise ratio. 3-D acquisitions may be possible, which can simplify the examination by removing the need for acquisition of additional 2-D image sequences in different planes. 3-D acquisitions have been shown to have comparable accuracy to 1.5-T 2-D image acquisitions. In the authors’ experience, 3-T imaging provides increased signal-to-noise ratio in obtaining high-resolution images, which has been substantiated in the evaluation of other pelvic tumors. Imaging parameters should be adjusted accordingly for 1.5-T or 3-T systems.
Endorectal coils provide high-resolution images that are able to delineate bowel wall layers. Some investigators have reported superior diagnostic accuracy compared with phased-array coils alone for T staging, with sensitivity reaching 100% and specificity of 86%. As with endorectal ultrasound, there are several drawbacks of a primary endorectal MR imaging technique as well as increased cost and examination time. Developments in MR imaging phased-array coil technology have enabled high spatial resolution, high-contrast resolution scanning, which provides information comparable to that obtained with an endorectal coil. Endorectal coils are thus not used routinely in practice for rectal cancer staging.
The mainstay of MR evaluation for rectal cancer is T2 fast spin-echo imaging. An initial sagittal localizer sequence should be obtained from sidewall to sidewall, which identifies the primary tumor. This sequence is also used for planning high-resolution T2 axial images orthogonal to the rectum involved by tumor. Subsequently, large FOV axial and coronal T2 sequences of the entire pelvis should be obtained. Involvement of the peritoneal reflection and pelvic sidewalls is most clearly depicted on sagittal and coronal images. Triplanar imaging is of particular utility in the assessment of large or tortuous tumors where positioning of a single axial plane is difficult. Suzuki and colleagues have found that using a protocol, including triplanar T2 sequences with high-resolution imaging, resulted in significantly better correlation with histopathology regarding anterior organ involvement (sensitivity 86 vs 50% and specificity 94 vs 33%).
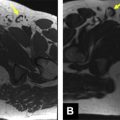
High-resolution T2 thin-section sequence with smaller FOV of 16 to 20 cm and slice thickness of 3 mm should be obtained through the primary tumor. Proper planning of high-resolution T2 imaging sequences is essential to staging accuracy. The initial sagittal sequence is used for planning of the orthogonal plane T2 high-resolution images. Determining the site of tumor origin from the rectal wall allows for correct positioning, the orthogonal plane. This sequence must be perpendicular to long axis of rectum at the level of tumor because it provides the most accurate evaluation of tumor, depth of mural or transmural invasion, and distance from the CRM, as shown in Fig. 2 . Obtaining images not perpendicular to the axis of tumor may result in misinterpretation due to volume averaging; for example, blurring of muscularis propria or pseudospiculated appearance that could lead to overstaging. In bulky tumors or if there is tortuosity of the colon, it may be necessary to obtain images at multiple angles.
In patients with low rectal cancers, high spatial resolution coronal oblique T2 through the sphincter complex depicts the relationship of the tumor to the rectal wall. This sequence provides information on intersphincteric plane and levator muscle involvement.
Conventional T1 images are not helpful for bowel wall layer depiction, because of similar relaxation rates of bowel wall and tumor. T1 imaging primarily provides information about pelvic bones and, in some cases, nodal anatomy.
The addition of diffusion-weighted images (DWIs) to T2-weighted images improves accuracy for rectal cancer detection. Images are acquired in the axial plane with the patient in free breathing, from the level of the aortic bifurcation to the upper thigh, in order to include inferior mesenteric lymph nodes and groin nodes. In the authors’ practice, diffusion gradients at 4 time points (or b values) are obtained (b values 0, 50, 400, and 800). An apparent diffusion coefficient (ADC) map is subsequently generated using all b values. Low b-value images (0 and 50) provide maximal lesion detection, particularly for the presence of lymph nodes and bone metastases, whereas high b-value images (b value 800) provide signal suppression of highly cellular structures, such as gastrointestinal and urogenital lining, to maximize conspicuity of tumor. The high signal intensity focus depicting a tissue with restricted diffusion is readily apparent against a low signal intensity background of bowel wall and feces on high b-value images. Hence, the sequence generally aids in detection of small tumors not seen on T2 images.
DWI has several limitations. Accuracies for tumor detection have been reported up to 90%, but benign tumors may show restricted diffusion. Also, the lower spatial resolution of DWI sequences may make it more difficult to detect small lesions. False-positive results may result from air-filled colon and from slice thickness in small tumors. Hyperintensity due to collapsed bowel wall may also mimic disease. For this reason, it has been suggested that evaluation of ADC may be more reliable, although some investigators think that ADC evaluation is cumbersome in practice.
DWI is highly sensitive in nodal detection but has limited value for characterizing lymph nodes, because there is significant overlap in ADC values for benign and malignant nodes. A recent study performed by Mizukami and colleagues comparing DWI and conventional MR assessment to histopathologic specimen found that the node based sensitivity is 97% and negative predictive value 84%, whereas specificity was much lower (81%), resulting in a positive predictive value of only 52%.
Gadolinium contrast has not proved effective for rectal cancer staging. Jao and colleagues have found that gadolinium-enhanced T1-weighted MR imaging does not increase the diagnostic yield for tumor and nodal staging and can be omitted from the staging protocol. It has also been shown that contrast enhancement does not improve diagnostic accuracy for assessment of tumor penetration through rectal wall and tumor extension into MRF. Dynamic imaging is used, however, as part of the authors’ routine multiparametric protocol to increase confidence levels for detection of small tumors, characterizing nodes, assessing treatment response, and evaluating any incidental pelvic or bone abnormalities detected during the examination ( Table 1 ).
| MR Imaging Sequence | ||||||
|---|---|---|---|---|---|---|
| Sagittal T2 FSE | Axial T2 FSE | Coronal T2 FSE | Oblique Hi-res T2 FSE | DWI | T1 FSPGR | |
| Repetition time (ms) | 3500 | 3320 | 3500 | 4000 | 5800 | 4.44 |
| Echo time (ms) | 91 | 91 | 91 | 80 | 96 | 1.59 |
| Number of slices | 28 | 40 | 25 | 15 | 30 | 32 |
| Bandwidth (Hz/Px) | 391 | 391 | 391 | 391 | 1132 | 400 |
| FOV (mm) | 220 | 220 | 220 | 200 | 250 | 240 |
| Slice thickness (mm) | 3 | 4 | 4 | 3 | 4 | 4 |
| Distance factor (%) | 25 | 25 | 25 | 0 | 20 | 20 |
| Phase FOV (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Number of acquisitions | 3 | 2 | 2 | 3 | 6 | 1 |
| Matrix | 350 × 263 | 350 × 263 | 350 × 263 | 350 × 263 | 250 × 250 | 240 × 240 |
| Phase encode direction | Anterioposterior | Transverse (R>L) | Transverse (R>L) | Anterioposterior | Anterioposterior | Anterioposterior |
| Saturation band | Anterior | N/A | N/A | Superior and inferior | N/A | N/A |
| Acquisition time (minutes) | 4 | 5.5 | 4 | 5 | 4.5 | 1 |
| Base resolution | 320 | 320 | 320 | 320 | 192 | 320 |
| Voxel size (mm) | 0.7 × 0.7 × 4.0 | 0.7 × 0.7 × 4.0 | 0.7 × 0.7 × 4.0 | 0.6 × 0.6 × 3.0 | 1.7 × 1.3 × 4.0 | 0.9 × 0.8 × 4.0 |
Relevant anatomy
Anatomic landmarks important to rectal cancer surgery may be defined on MR imaging, which is of use in staging tumors, assessing resectability, planning surgery, and selecting patients for preoperative neoadjuvant therapy.
The anal verge is an important surgical landmark, because it is easily identified at physical examination. It marks the most distal aspect of the anal canal. The location of the lower border of the tumor should be determined relative to this line, which is most easily depicted on sagittal images ( Fig. 3 ).
The anal canal comprises an internal sphincter and external sphincter complex, separated by a thin, fat-containing intersphincteric plane ( Fig. 4 ). The internal sphincter is a continuation of the smooth muscle layer of the rectum. The external sphincter complex begins cranially at the inferior insertion of levator ani muscles and includes the puborectalis muscle and the more inferior external sphincter muscles, shown in Fig. 4 . The anatomic relationship of the most inferior aspect of the tumor for the top border of the anal sphincter (ie, puborectalis) and the depth of involvement of tumor is of critical importance in MR imaging evaluation, because it identifies patients who may not be candidates for sphincter preservation surgery.
Identification of rectal wall layers allows for accurate T staging. The outer muscular layer is readily apparent by MR imaging as a hypointense line. The inner mucosa and submucosa are indistinguishable as a thicker band of slightly higher signal intensity. Perirectal fat is identified as high signal intensity surrounding the outer muscular layer.
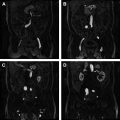
The anterior peritoneal reflection separates the intra- and extraperitoneal portions of the rectum and is a well-defined anatomic landmark at laparotomy. Experienced radiologists identify the anterior peritoneal relection in more than 80% of cases. In the midsagittal plane, the anterior peritoneal reflection consisted of a thin T2 hypointense line 1 mm or less in thickness on most MR imaging studies ( Fig. 6 ). The peritoneum extends over the surface of the bladder posteriorly to the point of attachment at the junction of the upper two-thirds and lower one-third of the rectum. In men, the tip of the seminal vesicles is a consistent landmark for the location of the most inferior portion of the peritoneal membrane. In women, the location is variable, but the reflection is commonly seen at the uterocervical angle. A trace amount of fluid in the pelvic cul-de-sac increases the conspicuity of the anterior peritoneal reflection. The peritoneum attaches in a V-shaped manner onto the anterior aspect of the rectum ( Fig. 5 ), an appearance characterized by Brown and colleagues as the seagull sign . The peritoneum-lined recess between the rectum and the posterior aspect of the bladder is termed, the rectovesical pouch . Proper assessment of the anterior peritoneal reflection requires evaluation of both axial and sagittal images. The ability to visualize the anterior peritoneal reflection and its relationship with rectal tumor on MR imaging has implications for spread of tumor and allows for further individualization of treatment.
MRF is consistently visualized on MR imaging as a distinct thin T2 hypointense layer surrounding the mesorectum and is best seen on axial images (see Fig. 6 ). It is difficult to recognize the MRF at the distal and anterior portions of the rectum because of the small amount of fat tissue.
The rectoprostatic fascia, or Denonvilliers fascia, is a focal thickening of the MRF at midline anteriorly that separates the prostate and urinary bladder from the rectum. It is a single fibromuscular structure that contains several fused layers ( Fig. 7 ).
Imaging findings
T Staging
Goals of preoperative imaging in rectal cancer include accurate tumor localization, sphincter involvement, depth of mural involvement and extramural extension into mesorectal fat, evaluation of MRF for threatened resection margin, status of peritoneal reflection, and identification of extramural vascular invasion (EMVI) and nodal metastases ( Table 2 ).
| TNM Staging of Rectal Cancer | |||
|---|---|---|---|
| T stage | Tx | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1 | Tumor invades submucosa | ||
| T2 | Tumor invades muscularis propria | ||
| T3 | Tumor invades through muscularis propria to pericolorectal tissues | ||
| a | Tumor <5 mm into the perirectal fat or extramural | ||
| b | Tumor 5–10 mm into the perirectal fat or extramural | ||
| c | Tumor >10 mm into the perirectal fat or extramural | ||
| T4 | Organ invasion | ||
| a | Tumor penetrates to surface of visceral peritoneum | ||
| b | Tumor directly invades or is adherent to other organs or structures | ||
| N stage | Nx | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in 1–3 regional lymph nodes | ||
| N2 | a | Metastasis in one regional lymph node | |
| b | Metastasis in 2–3 regional lymph nodes | ||
| M stage | M0 | No distant metastais | |
| M1 | a | Metastasis confined to one organ or site | |
| b | Metastasis in more than one organ or site or peritoneum | ||
For purposes of MR reporting, the rectum is divided longitudinally into thirds ( Fig. 8 ). A tumor is located in the upper third of the rectum if it is more than 10 cm from the anal verge. Because tumors at this level are generally covered anteriorly by the anterior peritoneal reflection, the relationship to the peritoneal reflection (eg, above, straddles, or below) at this level is important. A tumor arising in the midthird of the rectum is between 5 and 10 cm from the anal verge, where the rectum is usually encircled by mesorectal fat. A tumor is located in the lower third if it is less than 5 cm from the anal verge. Tumors residing here may be at or below the sphincter complex, requiring special considerations for MR reporting. Craniocaudal location of rectal tumor is frequently significant for surgical planning. Patients with tumors in the mid- and high-rectum are usually candidates for sphincter-sparing surgery, whereas patients with low rectal tumors have variable candidacy for sphincter preservation.
Thin-section, high-resolution T2 images in a plane perpendicular to the axis of the involved rectum are highly accurate for evaluation of tumor extent. Tumor demonstrates intermediate T2 intensity, distinct from the hyperintense submucosal layer and hypointense muscular layer ( Fig. 9 ).
T1 Lesions
T1 lesions confined to submucosa are seen at MR imaging as hypointense to the surrounding submucosa. Because there is no penetration to the muscular layer, a discrete intact hyperintense ring of submucosa deep to the deep margin of the tumor may be visible. The intact submucosal layer is variably present and visualization is not necessary for the diagnosis of a T1 tumor. T1 tumors may be difficult to accurately stage with MR imaging, and phased array coil MR imaging may not reliably differentiate T1 and T2 tumors.
T2 Lesions
T2 lesions extend into the hypointense muscularis propria layer, without breaching the outer margin. At MR imaging, tumor is seen as intermediate signal (higher than muscle and lower than submucosa) that does not extend beyond the outer margin of the muscular layer (see Fig. 9 ).
T3 Lesions
T3 tumors are transmural in extent and involve mesorectal fat but do not involve the serosal surfaces or invade adjacent structures. In general, the extension beyond the muscular layer has a broad-based nodular configuration within the perirectal fat. Continuity of tumor signal in perirectal fat with the intramural portion of the tumor is crucial. Disruption in the outer muscular layer does not necessitate tumor invasion, because small penetrating wall vessels may give this appearance. Thin spiculations may also represent peritumoral fibrosis and are not sensitive or specific in the diagnosis of T3 lesions. Tumoral nodules within mesorectal fat separate from the tumor itself are also considered as T3 stage.
T2 Versus Early T3 Lesions
The ability to distinguish T2 from early T3 lesions lies in the distinction of spiculation of perirectal fat due to peritumoral fibrosis from fibrosis with tumor infiltration. MR imaging does not differentiate well between T2 and early T3 lesions, but this distinction is unlikely to be of clinical significance because patients with early T3 lesions receive little benefit from preoperative neoadjuvant therapy. In tumors where there is spiculation of the mesorectal fat, making assessment difficult, reporting tumor as T2/early T3 allows the multidisciplinary team to tailor therapy in the overall assessment of the patient ( Figs. 10 and 11 ).