Magnetic Resonance (MR) imaging is the most sensitive modality for breast cancer detection but is currently limited to screening women at high risk due to limited specificity and test accessibility. However, specificity of MR imaging improves with successive rounds of screening, and abbreviated approaches have the potential to increase access and decrease cost. There is growing evidence to support supplemental MR imaging in moderate-risk women, and current guidelines continue to evolve. Functional imaging has the potential to maximize survival benefit of screening. Leveraging MR imaging as a possible primary screening tool is therefore also being investigated in average-risk women.
Key points
- •
Magnetic resonance (MR) imaging has a modality-based advantage compared to mammography and sonography in early detection of invasive breast cancer, which is being leveraged to optimize screening outcomes.
- •
Supplemental screening with MR imaging has been found to be of value in high-risk women as well as in certain subgroups of higher-than-average-risk women, but careful cost-benefit considerations are needed.
- •
Overall adherence to MR imaging among currently eligible women is poor even as screening indications of MR imaging continue to evolve.
Introduction
Magnetic resonance (MR) imaging is an advanced modality currently reserved for supplemental breast cancer screening in high-risk individuals, with excellent sensitivity and specificity reported in recent literature. Although MR imaging–specific impact on breast cancer mortality is difficult to assess, supplemental screening with MR imaging has been associated with detection of earlier-stage disease and improved 10-year survival. , Although mammography is the standard of care for population-wide screening known to decrease mortality, questions of overdiagnosis and overtreatment persist. With increasingly sophisticated understanding of breast cancer heterogeneity and outcomes based on cancer subtypes, there is growing impetus to parse out modality-based cancer yield both in number and in the type of cancers detected. The advantage of contrast-enhanced MR imaging as a functional imaging modality optimized to capture biologically more aggressive tumors that may be mammographically occult is the basis of a growing interest in expanding the role of MR imaging in breast cancer screening. Furthermore, there is growing evidence that MR imaging also outperforms mammography and sonography in moderate-risk women in cancer yield, prompting more recent broadening of MR imaging screening indications in certain guidelines. However, patient access to MR imaging remains limited, and the cost and time currently associated with the examination can be prohibitive. Efforts to improve feasibility of wider implementation have focused on streamlining examination acquisition and interpretation while preserving diagnostic accuracy. This article therefore (1) provides an evidence-based overview of current MR imaging screening indications, (2) considers the rationale for expanding its use in the population, and (3) discusses challenges and potential solutions in improving its cost-effectiveness with abbreviated approaches, notably via abbreviated and ultrafast MR imaging protocols.
Current magnetic resonance imaging screening indications
High-Risk Screening
Women with an estimated lifetime risk (LTR) of greater than or equal to 20% to 25% for developing breast cancer are defined as high risk as per the American Cancer Society (ACS) guidelines. A woman’s LTR is usually estimated based on family history and risk modeling algorithms. There is well-established evidence supporting MR imaging screening in this group, for whom annual supplemental MR imaging in addition to mammography is currently the standard of care in breast cancer screening.
Hereditary and Familial Risks
Although there are variations in what constitutes high risk across multidisciplinary guidelines, BRCA germline mutation carriers and untested first-degree relatives are universally acknowledged as harboring risks an order of magnitude greater than that of the general population, and are thought to benefit the most from MR imaging screening , , (see Table 2 ). Pooled data have shown that the average risk of developing breast cancer by age 70 years is 65% for BRCA1 mutation carriers and 45% for BRCA2 mutation carriers. Compared with women without mutations, BRCA1 and BRCA2 carriers have, on average, respectively 30-fold and 10-fold to 16-fold higher LTR of breast cancer. In women with BRCA mutations who undergo screening, mammography has low sensitivity because of high breast density and more rapidly growing tumors in younger women. Prospective trials have shown that annual supplemental MR imaging in conjunction with mammography typically doubles the sensitivity of mammography alone and generally achieves sensitivities greater than 90%. , , , , , Further ultrasonography or clinical breast examination are not additive ( Table 1 ). False-positive rates are increased with addition of screening MR imaging to annual mammography, but these false-positives tend to decrease in successive incident rounds of screening. In a multicentered prospective trial from the Netherlands, the sensitivity advantage of MR imaging versus mammography was greatest at prevalent round (93% vs 20%; P = .003) but maintained in subsequent rounds (77% vs 29%; P = .02); and the false-positive rate of MR imaging decreased in subsequent rounds (from 14% to 8%). MR imaging–detected cancers also have a favorable stage distribution in BRCA mutation carriers, primarily composed of sub–1-cm invasive cancers and in situ cancers at incident rounds of screening with low rates of node positivity (12%–26%), associated with improved 10-year survival. Importantly, timing the frequency of screening has been a challenge in this population. Particularly in BRCA1 carriers less than 50 years of age, who are known to have the highest rate of interval cancers because of a high prevalence of rapidly growing tumors, annual imaging may not be adequate. The main strategy has been to shorten the screening interval to every 6 months using various examination combinations, such as staggering mammography and MR imaging, or using biannual MR imaging with annual mammography, or by supplementing annual mammography/MR imaging with biannual ultrasonography. , MR imaging screening is generally deemed cost-effective for BRCA carriers assuming perfect attendance, particular for BRCA1 carriers, and adherence to the examination is high among carriers confirmed by genetic testing (80%–90%). ,
| Reference | Patients (n) | Rounds (n) a | Inclusion | Sensitivity | Specificity | PPV 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR Imaging (%) | MG (%) | US (%) | MR Imaging (%) | MG (%) | US (%) | MR Imaging (%) | MG (%) | US (%) | Interval CA (n) | ||||
| 2020 | 8782 | 20,053 | BRCA+/Fam | 91 | 41 | NA | 87 | 92 | NA | 20 | 26 | NA | 12 |
| 2019 , b | 674 | 2812 | Fam | 98 | 87 | NA | 84 | 91 | NA | 27 | 28 | NA | 1 |
| 2017 | 296 | 1170 | BRCA+/Fam | 68 | 37 | 32 | 95 | 98 | 95 | 25 | 34 | 10 | 3 |
| 2015 | 559 | 1506 | BRCA+/Fam | 90 | 38 | 38 | 89 | 97 | 97 | 20 | 28 | 27 | 1 |
| 2014 | 221 | 1855 | BRCA+ | 100 | 27 | 77 | 56 | 82 | 84 | NA | NA | NA | 1 |
| 2012 | 612 | 612 | Mixed/Dense | 88 | 52 | 45 | 76 | 91 | 90 | 23 | 38 | 12 | 9 |
| 2011 | 501 | 1592 | BRCA+/Fam | 91 | 50 | 52 | 97 | 99 | 98 | 56 | 71 | 62 | 3 |
| 2010 | 687 | 1679 | BRCA+/Fam | 93 | 33 | 37 | 98 | 99 | 98 | 48 | 39 | 36 | 0 |
a Number of rounds of screening indicates rounds that specifically include MR imaging.
b Randomized controlled study comparing MR imaging versus mammography.
Annual breast MR imaging is also recommended in less common mutations associated with high risk of breast cancer, such as TP53 (Li-Fraumeni syndrome; 95% by age 90 years) and PTEN (Bannayan-Riley-Ruvalcaba syndrome, Cowden syndrome; 85% by age 80 years), and is increasingly considered in additional mutations associated with moderate to high risk of breast cancer, including CDH1, STK11, PALB2, CHEK2, ATM, BARD1, and NF1, with individual decisions often guided by family history because of a further increased risk. However, most women with a family history of breast cancer do not have an identified genetic mutation, and 15% of all breast cancers occur in this group. , Family history in these women therefore serves as the primary basis for calculating LTR via modeling algorithms, and is a direct indication for supplemental MR imaging screening if calculated risk exceeds 20%. In this group, MR imaging has been found to increase detection of early-stage cancer compared with mammography (14 per 1000 vs 5 per 1000 cancers; P <.0003). Notably, in contrast with BRCA carriers, women at high risk without BRCA mutations have been found to have significantly lower adherence to supplemental screening MR imaging. , There is also evidence that, although supplemental MR imaging is underused in high-risk women, many who undergo breast MR imaging may not be appropriately high risk. , For example, for those with family history who undergo multigene panel testing in recent years, unclassified genetic variations of uncertain significance (VUS) are increasingly encountered. Although work is being done to better classify these genetic variants to provide clinically actionable information, detection of any unknown variant (including BRCA1/2 VUS) is currently not an indication for intensified breast cancer screening.
Prior Chest Radiation
Women with prior childhood chest radiation are another group at high risk for developing breast cancer later in life, for whom annual screening MR imaging is consistently recommended ( Table 2 ). By age 50 years of age, 1 in 3 women with prior chest radiation (risk greatest if subjected to ≥20 Gy) are diagnosed with breast cancer, a risk on par with that of BRCA1 mutation carriers. , Multiple studies have shown increased sensitivity of both mammography and MR imaging in this group (94%–100%), with supplemental MR imaging yielding additional cancers. Therefore, annual screening mammography and breast MR imaging are recommended, starting at age 25 years or 8 years following chest radiation, whichever occurs last. However, breast cancer screening adherence among childhood cancer survivors is poor. Notably, mammography screening adherence in women who have received chest radiation is lower than among their average-risk peers in the general population. Nearly half of survivors younger than 40 years of age have never obtained a mammogram, and only 52% of women aged 40 to 50 years undergo regular mammography screening. Adherence for MR imaging screening is markedly worse, and efforts to improve adherence have had marginal effects. In a recent randomized controlled study, although educational interventions increased the rate of uptake of mammography screening (from 18% to 33%), MR imaging uptake was not significantly changed (from 13% to 16%), and overall screening adherence in this population remains poor. Primary barriers identified by women survivors to completing screening included lack of physician recommendation, deferred action by survivor, absence of symptoms, and cost, which is a concern specific to MR imaging.
| Organization | BRCA Carriers/First-Degree Relatives a | Family History | Prior Radiation | Personal History | Dense Tissue | History of Atypia b |
|---|---|---|---|---|---|---|
| ACS 2007 | BRCA1/2/select mutations | If LTR ≥20% | Age 10–30 y | NR | NR | NR |
| ACR 2018 | BRCA1/2/select mutations | If LTR ≥20% | Age<30 y | If early diagnosis (before age 50) | If personal history (prior breast cancer) | If other risk factors |
| ASBrS 2019 | BRCA1/2/select mutations | If strong family history | Age 10–30 y | If early diagnosis (before age 50 y) | If personal history (prior breast cancer) | NR |
| NCCN 2020 | BRCA1/2/select mutations | If family history suggests hereditary pattern despite absence of mutation (eg, early diagnosis before age 30 y) | Age<30 y | NR | NR | If LTR ≥20% |
| EUSOBI 2015 | BRCA1/2/select mutations | Selective | Age<30 y | NR | NR | NR |
| ECIBS 2020 c | NS | NR | NS | NS | NR | NR |
| ACOG 2017 | BRCA1/2/select mutations | If LTR ≥20% | Age 10–30 y | If other risks | NR | NR |
a MR imaging is consistently recommended for BRCA mutation carriers and untested first-degree relatives, but more variably recommended or considered for other mutations.
b Atypia refers to atypical epithelial hyperplasia, including atypical lobular hyperplasia, lobular carcinoma in situ, and atypical ductal hyperplasia.
c The ECIBS guidelines primarily address average-risk women that attend organized screening programs in Europe but also include select higher-than-average-risk groups such as those with family history or high breast tissue density.
Evolving magnetic resonance imaging screening indications
Moderate-Risk Screening
Despite a lack of consensus among different guidelines, there is increasing evidence supporting expanding the indications of MR imaging screening to include women at intermediate risk for breast cancer, as defined by an estimated LTR of 15% to 20% as per the ACS. In particular, several subgroups of women with higher-than-average risk for breast cancer are considered, including those with a personal history of breast cancer, dense breast tissue, or a history of atypical epithelial hyperplasia (atypical ductal hyperplasia [ADH], atypical lobular hyperplasia [ALH], and lobular carcinoma in situ [LCIS]). Although the 2007 ACS guidelines reported insufficient evidence to recommend for or against MR imaging screening in these groups, the more recent 2018 American College of Radiology (ACR) and 2020 National Comprehensive Cancer Network (NCCN) guidelines suggest considering MR imaging in some or all of these women, particularly in conjunction with other overlapping risk factors that may increase LTR to greater than 20% (see Table 2 ).
Breast Cancer Survivors
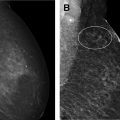
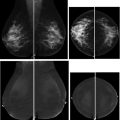
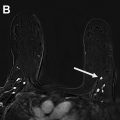
Women with a personal history of breast cancer are at considerable risk of developing a second breast cancer, with cumulative risks estimated at 5.4% in 5 years and 19.3% in 10 years following initial diagnosis. , In women diagnosed with breast cancer before age 50 years, the LTR for a second breast cancer is greater than 20%. Additional independent predictors of increased risk for a second breast cancer within 5 years also include aggressive tumor biology in the first cancer, treatment without radiation, and heterogeneously dense breasts on mammography. Therefore, the ACR Appropriateness Criteria currently recommend annual screening MR imaging in conjunction with mammography for women with breast cancer diagnosed before age 50 years, and for women with breast cancer history and dense breasts. Mammographic sensitivity is limited in the treated breast because of postsurgical parenchymal distortion, scarring, and fat necrosis. Although there are currently no prospective data, a growing body of retrospective studies have consistently shown superior sensitivities using MR imaging compared with mammography in women with a personal history of breast cancer (80%–100% vs 0%–53%), , and supplemental MR imaging in this group has been shown to perform as well as, if not better than, in those with genetic and familial predispositions ( Fig. 1 ). The 2018 ACR recommendations aim to optimize early detection of second breast cancers and improve survival but have not been universally adopted across multidisciplinary guidelines, pending data on mortality benefits, which are currently unknown (see Table 2 ).