This article explains the pathogenesis of fistula-in-ano and details the different classifications of fistula encountered, describe their features on MR imaging, and explains how imaging influences subsequent surgical treatment and ultimate clinical outcome. Precise preoperative characterization of the anatomic course of the fistula and all associated infection via MR imaging is critical for surgery to be most effective. MR imaging is the preeminent imaging modality used to answer pertinent surgical questions.
Key points
- •
Preoperative classification of fistula-in-ano is pivotal for successful surgical treatment because this determines what type of operation is performed.
- •
MR imaging is the most appropriate imaging modality with which to classify fistula-in-ano. It is more accurate than other imaging modalities and is even more accurate than examination under anesthetic overall.
- •
The major therapeutic impact of MR imaging lies with its ability to both identify the course of the fistula (thereby indicating the extent of any sphincter division) and identify areas of infection that would have been missed otherwise.
Introduction
Fistula-in-ano describes an abnormal communication between the anal (occasionally rectal) lumen and skin, usually perianal. Fistula-in-ano is relatively common and frequently recurs in the face of apparently curative surgery. Recurrence is usually due to infection that has escaped surgical detection, thereby going untreated. It is now well-recognized that preoperative MR imaging identifies fistulas and associated abscesses and extensions that would have been missed otherwise. Because of this, preoperative MR imaging has been shown to influence subsequent surgery and thereby diminish the chance of recurrence significantly. Preoperative MR imaging is routine in many centers, especially for patients with recurrent fistulas.
Etiology, classification, and treatment of fistula-in-ano
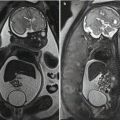
Cryptogenic fistula-in-ano is caused by chronic intersphincteric sepsis. Infection of the anal glands, which arise at the level of the dentate line (the squamocolumnar junction of the anal canal) is believed to be the initiating factor. An intersphincteric abscess results, that is, an abscess in the gap between the internal and external sphincter. Acute glandular infection often presents as an acute perianal abscess, which is easy to diagnose clinically, and familiar to any surgical resident. Although speedy incision and drainage provides substantial symptom relief, because the abscess is underpinned by glandular infection and the gland drains to the anal lumen, patients frequently develop a subsequent fistula. Of 165,536 patients presenting with acute abscess, 16% developed a fistula subsequently, increasing to 42% if there was inflammatory bowel disease ; fistulas usually developed within the first year. Acute MR imaging (before incision and drainage) may show intersphincteric infection, thus identifying cryptoglandular infection as the cause ( Fig. 1 ). A Cochrane review found that treatment of any underlying fistula found during surgery for acute abscess decreased the chance of recurrence substantially.

If intersphincteric sepsis is left untreated, it becomes chronic and burrows its way out, appearing as an external perianal opening. The anatomic course of the fistula is dictated by the location of the infected anal gland and the anatomic planes and structures that surround it. The internal opening is usually within the anal canal at the level of the dentate line, that is, the original site of the duct draining the infected gland. Radially, this is usually posterior at 6 o’clock, simply because anal glands are more abundant here, especially in men. The dentate line cannot be identified as a discrete anatomic structure by any imaging technique, but its position can be approximated with sufficient accuracy by experienced radiologists—it lies approximately 2 cm cranial to the anal verge.
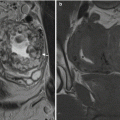
The fistula can reach the skin via a variety of routes, some more tortuous than others and penetrating anal sphincter muscles and surrounding tissues to a variable degree. Fistulas are classified according to the route taken by this primary track, that is, the main track that links the internal and external openings. There are many classification systems, both clinical and radiologic, but the only system recognized commonly by surgeons is from Parks and colleagues in 1976. Generally speaking, Parks’ is the only system that surgeons use, which obliges radiologists to use it as well. Parks and colleagues analyzed 400 consecutive fistula patients referred to St. Mark’s Hospital London, a specialist hospital dealing with coloproctological disease and found that all fistulas could be placed into 1 of 4 broad groups. They termed these: intersphincteric, trans-sphincteric, suprasphincteric, and extrasphincteric ( Fig. 2 ). The first 3 types pivot on intersphincteric infection, explained by the cryptoglandular hypothesis. The fourth type, extrasphincteric, does not exhibit intersphincteric infection and usually arises from primary disease beyond the sphincter complex, for example, rectal Crohn’s disease or sigmoid diverticulitis. MR imaging has been adopted widely simply because it provides accurate preoperative classification for the surgeon. It is worth noting that, if either the external or internal opening is absent, then “sinus” is the correct description.

Although most fistulas probably start as a single primary track, unabated infection may result in ramifications (often multiple) that branch away from this, generally termed extensions. Extensions may be intersphincteric, ischioanal, or supralevator (pararectal), with either track or abscess morphology. Exactly when a track becomes an abscess and vice versa has no precise definition on imaging and the surgical community also has no generally accepted definition. The ischioanal fossa is the commonest site for an extension, especially those arising from the apex of a trans-sphincteric fistula. The ischioanal fossa lies lateral to the sphincter complex and is largely fat filled. Because this compartment lies adjacent to the anus (vs the rectum) and lies below the pelvic floor (defined by the levator plate), the author prefers the term ischioanal fossa rather than ischiorectal, which surgeons use commonly. The 2 terms are largely interchangeable. Extensions also occur in the horizontal plane, and are known as horseshoes only when there is ramification of sepsis on both sides of the internal opening. Extensions are the prime cause of recurrent disease because they are missed frequently, often left untreated, and thereby allow infection to continue. Supralevator extensions are a particular problem because the levator plate presents a relative barrier to free drainage, so that infection may persist even when the extension has been identified and incised.
So, the surgeon’s prime objective is to identify the primary track and any associated extensions, eradicate these by draining all associated infection, and simultaneously preserving anal continence via minimal sphincter division. The surgeons needs answers to the following 2 questions:
- •
What is the relationship between the fistula and the anal sphincter? Will my surgery risk incontinence?
- •
Are there any extensions from the primary track that need to be treated to prevent recurrence? If so, where are they?
Surgical treatment is usually straightforward. Laying open the fistula, a procedure known as a fistulotomy, is the obvious surgical option, but risks incontinence if a substantial proportion of sphincter muscle is divided. Fistulotomy is therefore preceded by examination under (general) anesthetic (EUA), during which the surgeon attempts to classify the fistula via palpation and probing the external opening. This is not as easy as it sounds: The surgeon cannot visualize underlying muscles without incision, the internal opening may be very small and difficult to identify, the fistula may be very tortuous, and extensions may travel far from the external opening, among other considerations. Furthermore, injudicious, overenthusiastic probing can convert a simple fistula into a disaster. For example, probing the apex of a trans-sphincteric fistula could rupture through the levator plate, thereby causing a supralevator extension. Even worse, the probe can rupture into the rectum, causing an extrasphincteric fistula.
So, fistula classification by EUA alone can be very difficult, presenting ample opportunity to make matters worse. Patients with recurrent disease are particularly difficult: They are most likely to harbor extensions, but also the most difficult to assess because multiple failed previous operations results in scarring and induration that frustrates palpation. It can be extremely difficult to differentiate between an abscess or scar tissue on the basis of palpation alone. Furthermore, this group are also most likely to have extensions very remote from the primary track, frustrating their detection further. The more chronic the fistula, the more complicated the associated extensions tend to be. The inevitable result is that these patients become progressively more difficult to treat, with both patient and surgeon becoming ever more exasperated. The key to breaking this loop is accurate preoperative imaging.
If imaging suggests that extensive sphincter division will be necessary to lay open the fistula, then procedures aiming to minimize sphincter division can be deployed, but these often have a greater chance of postoperative recurrence. A variety are now available: Mucosal advancement flap is the most established and involves closing the internal opening using rectal mucosa. Newer techniques include ligation of the intersphincteric fistula tract and video-assisted anal fistula treatment, which aim to close the internal opening and eradicate intersphincteric infection while avoiding sphincter division. Plugs and glue have also been inserted into the fistula to try and achieve closure without surgical incision.
Imaging fistula-in-ano: which technique to use?
Before MR imaging, radiologists trying to answer the surgical questions posed elsewhere in this article met with little success. Contrast fistulography involves catheterizing the external opening with a fine cannula and injecting water-soluble contrast. It suffers 2 major drawbacks: First, extensions may fail to fill with contrast if they are plugged with debris, very remote, or if there is excessive contrast reflux from the internal and/or external openings. Second, the sphincter muscles and pelvic floor are not imaged directly, which means that the relationship between these and the fistula is largely conjecture. For example, it is frequently very difficult to decide whether a visualized extension is supralevator or in the roof of the ischioanal fossa (ie, infralevator). Ultimately, fistulography is difficult to interpret and unreliable. Initial reports of computed tomography scans were encouraging, but simple identification of the fistula is insufficient; correct classification is key. Compared with MR imaging, computed tomography scans suffer insufficient tissue contrast, especially if the fistula has not been cannulated, and its multiplanar capability also lags behind.
Anal endosonography (AES) was the first technique to visualize the anal sphincter complex with high spatial resolution and, naturally, AES has been applied to fistula classification. Although AES can be very useful, accurate interpretation depends highly on the experience of the sonographer. Also, structures remote from the endoluminal transducer are difficult to image because of limited beam penetration, especially if high frequency. Accordingly, extensions beyond the sphincter complex are missed easily and false-positive results also occur. For example, AES cannot differentiate infection or pus from fibrosis reliably, because all appear hyporeflective. This causes particular difficulties in patients with recurrent disease, because infected tracks and fibrotic scars frequently occur together. Although there is no doubt that AES is valuable in the right hands, MR imaging is superior: A study comparing AES to digital evaluation and MR imaging in 108 primary tracks found that digital evaluation in clinic correctly classified 61%, AES 81%, and MR imaging 90%. Although AES was particularly adept at predicting the site of the internal opening correctly, there is little doubt that MR imaging is a superior technique overall and is certainly more generalizable.
MR Imaging Technique
MR imaging has emerged as the preeminent imaging modality to classify fistula-in-ano because it can depict infected tracks and extensions vividly and define their relationship to surrounding structures precisely. It is straightforward to image in the surgically relevant coronal plane and fistula morphology can be determined easily. The ability of MR imaging to not only accurately classify tracks but also to identify disease that would otherwise have been missed has had a palpable effect on surgical treatment and, ultimately, patient outcome ; this factor underpins its widespread adoption.
Field strength is not critical and excellent results can be obtained using relatively modest MR imaging scanners without specialized coils. Phased array surface coils increase the signal-to-noise ratio and spatial resolution to good effect, and are generally available. Although the best spatial resolution is achieved via dedicated endoluminal anal coils, these suffer the same limitation as AES—the limited field of view means that distant extensions are missed easily. They are also frequently uncomfortable for the patient. So, after initial enthusiasm, they are now used rarely. It should also be stressed that endoluminal anal coils are not rectal coils; they are smaller in diameter and their active element crosses the sphincter complex, not the rectal ampulla.
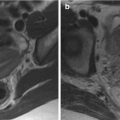
The MR imaging sequences used to classify fistula-in-ano must combine anatomic precision (to determine the course of the fistula with respect to adjacent structures), with the facility to highlight sepsis (usually pus) and tissue inflammation. Many investigators use widely available fast spin echo T2-weighted sequences, which highlight the hyperintense fluid within the fistula while simultaneously discriminating the individual layers of the anal sphincter complex. Fat suppression techniques are very useful. The earliest reports used short T1 inversion recovery (STIR) imaging, with the addition of T1-weighted scans to help anatomic clarification, and gadolinium contrast may be used if desired. Other approaches have included saline instillation into the external opening or rectal contrast medium, but such measures increase complexity in the face of already excellent results, so there is little motivation to adopt them. For the majority of his clinical work the Author uses either 1.5 T or 3.0 T magnets and STIR/T2-weighted sequences, which makes for a very rapid and easy examination. A typical examination would comprise sagittal and axial T2-weighted acquisitions with axial and coronal STIR acquisitions.
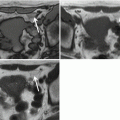
Perhaps more important than the sequences used is the need to align the scan planes with respect to the anal sphincter axis. This is because the anal canal is tilted forwards from the vertical by approximately 45°. Straight axial and coronal images with respect to the table will provide oblique images of the sphincter complex, making the geography of any fistula very difficult to ascertain. This is especially so when trying to determine the height of the internal opening. Oblique axial and coronal planes orientated orthogonal and parallel to the anal sphincter are fundamental, and planned easily from a midline sagittal image ( Fig. 3 ). It may be necessary to align supplementary scans to the rectal axis for complex extrasphincteric cases with high rectal openings, but this is rare.