Hepatic neoplasms constitute approximately 5% to 6% of all pediatric intra-abdominal masses, most of which are malignant. When compared with traditional multiphase computed tomography protocols that are often used in adults, magnetic resonance (MR) imaging is particularly desirable for evaluating liver lesions in children because of the lack of ionizing radiation and superb soft tissue contrast resolution. The goal of this article is to discuss common benign and malignant pediatric hepatic lesions and their key MR imaging findings. Particular emphasis is placed on the utility of new hepatocyte-specific contrast agents to narrow the differential diagnosis.
Key points
- •
Magnetic resonance imaging is a useful tool for characterizing both benign and malignant pediatric liver lesions.
- •
Characteristic patterns of signal intensity and postcontrast enhancement can narrow the differential diagnosis and help determine whether a liver lesion is likely benign or malignant.
- •
Use of hepatocyte-specific contrast agents, such as gadoxetate disodium, can provide additional information to help refine the differential diagnosis for focal liver lesions.
Introduction
Primary hepatic neoplasms constitute approximately 5% to 6% of all pediatric intra-abdominal masses, most of which (approximately two-thirds) are malignant. Overall, malignant primary hepatic tumors account for 1% to 2% of all childhood cancers. It is important, therefore, to systematically approach and accurately characterize hepatic masses to ensure the best possible clinical management strategy ( Table 1 ).
In children, magnetic resonance (MR) imaging is particularly desirable for evaluating liver lesions because of the lack of ionizing radiation and superb soft tissue contrast resolution compared with traditional multiphase computed tomography (CT) protocols. The potential disadvantages of sedation and scan time may be offset by the ability of MR imaging to provide more clinically relevant information when compared with CT. The recent development and implementation of hepatocyte-specific contrast agents (HSA), such as gadoxetate disodium, further strengthens the role of MR imaging in hepatic lesion characterization.
Thus, understanding how pathologic features of hepatic neoplasms are represented in imaging helps the radiologist to better characterize the lesions, which ideally leads to proper work-up and treatment of liver lesions. This article discusses key MR imaging findings of common benign and malignant pediatric lesions ( Tables 2 and 3 ) and focuses on the utility of new HSA to help achieve optimal diagnostic accuracy.
| Neoplasm | Ultrasound | CT | MR Imaging |
|---|---|---|---|
| Hypoechoic or mixed echogenicity Doppler shows increased diastolic flow | Peripheral early phase postcontrast enhancement with homogenous or centripetal fill in (note, larger lesions may not entirely fill in because of fibrosis or hemorrhage) | T2W hyperintense T1W hypointense Early phase postcontrast enhancement with homogenous or delayed fill in Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Mixed echogenicity depending on mixture of cystic and solid portions | Multicystic ± solid components that enhance with IV contrast material | Cystic areas are T2W hyperintense and T1W hypointense Solid portions enhance Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Hyperechoic, isoechoic, or hypoechoic Central scar is hyperechoic | Homogenous with arterial postcontrast enhancement Isoattenuating or nearly isoattenuating on noncontrast and delayed postcontrast imaging Central scar shows delayed enhancement | T1W hypointense/isointense T2W hyperintense/isointense T2W hyperintense central scar that shows delayed enhancement Arterial-phase postcontrast hyperenhancement Isointense on delayed venous imaging Hyperintense/isointense to normal liver on delayed imaging using HSA (20 min) |
| Hyperechoic, isoechoic, or hypoechoic depending on amount of lesional hemorrhage and lipid and underlying liver disease | Heterogeneous because of lipid and/or hemorrhage Variable heterogeneous postcontrast enhancement | Heterogeneously hyperintense on T1W and T2W because of hemorrhage and/or lipid Heterogeneous arterial-phase postcontrast enhancement Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Hyperechoic, isoechoic, or hypoechoic | Hyperenhancing or hypoenhancing (depending on postcontrast imaging phase) | Variable T2W signal intensity Isointense to hyperintense on T1W May show arterial postcontrast enhancement Hyperintense/isointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous (mixed echogenicity) Patchy or sparse Doppler blood flow | Heterogeneous postcontrast enhancement (enhancement may increase on delayed imaging) | Heterogeneous T1W and T2W signal intensity Heterogeneous postcontrast enhancement (enhancement may increase on delayed imaging) Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Neoplasm | Ultrasound | CT | MR Imaging |
|---|---|---|---|
| Heterogeneous Predominantly solid ± Calcification/ossification | Predominantly solid ± necrotic or cystic areas ± Calcification/ossification Heterogeneous postcontrast enhancement ± Vascular invasion | Heterogeneous T1W and T2W Heterogeneous postcontrast enhancement ± Vascular invasion Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous Predominantly solid | Predominantly solid May contain necrotic or cystic areas Postcontrast arterial-phase hyperenhancement with delayed wash out | Heterogeneous T1W and T2W Postcontrast arterial-phase hyperenhancement with delayed wash out ± Vascular invasion Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Solid, cystic, or mixed solid/cystic Generally multiple lesions | Solid, cystic, or mixed solid/cystic Generally multiple lesions Hypoenhancing or hyperenhancing on postcontrast imaging depending on tumor of origin and imaging phase | Solid, cystic, or mixed solid/cystic Generally T1W hypointense to isointense and T2W hyperintense Hypoenhancing or hyperenhancing on postcontrast imaging depending on tumor of origin and imaging phase Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Solid Central scar ± echogenic calcification/ossification | Solid Postcontrast arterial-phase hyperenhancement ± wash out Hypoenhancing central scar ± calcification | Solid Variable T1W and T2W T2W hypointense, hypoenhancing central scar Postcontrast arterial-phase hyperenhancement ± wash out Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous Predominantly solid | Commonly appears cystic and solid Heterogeneous postcontrast enhancement with septations and mural nodularity ± Hemorrhage | Commonly appears cystic and solid Variable T1W and T2W Heterogeneous postcontrast enhancement with septations and mural nodularity Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Solid, cystic or mixed solid/cystic Tumor may be observed in dilated bile ducts | Heterogeneous postcontrast enhancement Enhancing tumor may be observed in dilated bile ducts | T1W hypointense and T2W hyperintense Heterogeneous postcontrast enhancement Enhancing tumor may be observed in dilated bile ducts Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous Mixed solid and cystic | Mixed solid and cystic Heterogeneous postcontrast enhancement | Variable T1W and T2W Hypointense on all postcontrast phases, including delayed HAS imaging (20 min) |
Introduction
Primary hepatic neoplasms constitute approximately 5% to 6% of all pediatric intra-abdominal masses, most of which (approximately two-thirds) are malignant. Overall, malignant primary hepatic tumors account for 1% to 2% of all childhood cancers. It is important, therefore, to systematically approach and accurately characterize hepatic masses to ensure the best possible clinical management strategy ( Table 1 ).
In children, magnetic resonance (MR) imaging is particularly desirable for evaluating liver lesions because of the lack of ionizing radiation and superb soft tissue contrast resolution compared with traditional multiphase computed tomography (CT) protocols. The potential disadvantages of sedation and scan time may be offset by the ability of MR imaging to provide more clinically relevant information when compared with CT. The recent development and implementation of hepatocyte-specific contrast agents (HSA), such as gadoxetate disodium, further strengthens the role of MR imaging in hepatic lesion characterization.
Thus, understanding how pathologic features of hepatic neoplasms are represented in imaging helps the radiologist to better characterize the lesions, which ideally leads to proper work-up and treatment of liver lesions. This article discusses key MR imaging findings of common benign and malignant pediatric lesions ( Tables 2 and 3 ) and focuses on the utility of new HSA to help achieve optimal diagnostic accuracy.
| Neoplasm | Ultrasound | CT | MR Imaging |
|---|---|---|---|
| Hypoechoic or mixed echogenicity Doppler shows increased diastolic flow | Peripheral early phase postcontrast enhancement with homogenous or centripetal fill in (note, larger lesions may not entirely fill in because of fibrosis or hemorrhage) | T2W hyperintense T1W hypointense Early phase postcontrast enhancement with homogenous or delayed fill in Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Mixed echogenicity depending on mixture of cystic and solid portions | Multicystic ± solid components that enhance with IV contrast material | Cystic areas are T2W hyperintense and T1W hypointense Solid portions enhance Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Hyperechoic, isoechoic, or hypoechoic Central scar is hyperechoic | Homogenous with arterial postcontrast enhancement Isoattenuating or nearly isoattenuating on noncontrast and delayed postcontrast imaging Central scar shows delayed enhancement | T1W hypointense/isointense T2W hyperintense/isointense T2W hyperintense central scar that shows delayed enhancement Arterial-phase postcontrast hyperenhancement Isointense on delayed venous imaging Hyperintense/isointense to normal liver on delayed imaging using HSA (20 min) |
| Hyperechoic, isoechoic, or hypoechoic depending on amount of lesional hemorrhage and lipid and underlying liver disease | Heterogeneous because of lipid and/or hemorrhage Variable heterogeneous postcontrast enhancement | Heterogeneously hyperintense on T1W and T2W because of hemorrhage and/or lipid Heterogeneous arterial-phase postcontrast enhancement Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Hyperechoic, isoechoic, or hypoechoic | Hyperenhancing or hypoenhancing (depending on postcontrast imaging phase) | Variable T2W signal intensity Isointense to hyperintense on T1W May show arterial postcontrast enhancement Hyperintense/isointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous (mixed echogenicity) Patchy or sparse Doppler blood flow | Heterogeneous postcontrast enhancement (enhancement may increase on delayed imaging) | Heterogeneous T1W and T2W signal intensity Heterogeneous postcontrast enhancement (enhancement may increase on delayed imaging) Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Neoplasm | Ultrasound | CT | MR Imaging |
|---|---|---|---|
| Heterogeneous Predominantly solid ± Calcification/ossification | Predominantly solid ± necrotic or cystic areas ± Calcification/ossification Heterogeneous postcontrast enhancement ± Vascular invasion | Heterogeneous T1W and T2W Heterogeneous postcontrast enhancement ± Vascular invasion Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous Predominantly solid | Predominantly solid May contain necrotic or cystic areas Postcontrast arterial-phase hyperenhancement with delayed wash out | Heterogeneous T1W and T2W Postcontrast arterial-phase hyperenhancement with delayed wash out ± Vascular invasion Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Solid, cystic, or mixed solid/cystic Generally multiple lesions | Solid, cystic, or mixed solid/cystic Generally multiple lesions Hypoenhancing or hyperenhancing on postcontrast imaging depending on tumor of origin and imaging phase | Solid, cystic, or mixed solid/cystic Generally T1W hypointense to isointense and T2W hyperintense Hypoenhancing or hyperenhancing on postcontrast imaging depending on tumor of origin and imaging phase Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Solid Central scar ± echogenic calcification/ossification | Solid Postcontrast arterial-phase hyperenhancement ± wash out Hypoenhancing central scar ± calcification | Solid Variable T1W and T2W T2W hypointense, hypoenhancing central scar Postcontrast arterial-phase hyperenhancement ± wash out Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous Predominantly solid | Commonly appears cystic and solid Heterogeneous postcontrast enhancement with septations and mural nodularity ± Hemorrhage | Commonly appears cystic and solid Variable T1W and T2W Heterogeneous postcontrast enhancement with septations and mural nodularity Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Solid, cystic or mixed solid/cystic Tumor may be observed in dilated bile ducts | Heterogeneous postcontrast enhancement Enhancing tumor may be observed in dilated bile ducts | T1W hypointense and T2W hyperintense Heterogeneous postcontrast enhancement Enhancing tumor may be observed in dilated bile ducts Hypointense on delayed postcontrast imaging using HSA (20 min) |
| Heterogeneous Mixed solid and cystic | Mixed solid and cystic Heterogeneous postcontrast enhancement | Variable T1W and T2W Hypointense on all postcontrast phases, including delayed HAS imaging (20 min) |
Imaging technique/protocol
MR imaging has some distinct advantages compared with CT and ultrasound (US), which include lack of ionizing radiation (compared with CT), more specific soft tissue characterization, dynamic postcontrast imaging, and more complete evaluation of the biliary system. The lack of ionizing radiation becomes increasingly important when multiphase (including delayed phase) imaging is needed to assess the enhancement characteristics of focal lesions. MR imaging is particularly useful for the characterization of cystic and solid lesions, which are commonly indeterminate in nature at both CT and US, facilitating the formulation of a focused differential diagnosis. Another specific advantage of MR imaging compared with CT is with the evaluation of the biliary tree because bile duct masses, wall thickening, and filling defects are all better visualized with this modality.
Disadvantages of MR imaging include the length of scan time compared with CT or US and its sensitivity to patient motion, possibly necessitating sedation or general anesthesia in pediatric patients. Other disadvantages may include incompatibility with indwelling ferromagnetic devices; susceptibility artifacts and distortion from metallic implants, such as spinal hardware; and limited characterization of calcification/bone. In most clinical imaging situations, the benefits of MR imaging outweigh these disadvantages. The authors’ protocol for imaging of the pediatric liver is presented in Table 4 .
| Precontrast Imaging | Postcontrast Imaging |
|---|---|
| Coronal, single-shot fast spin echo (breath held or respiratory triggered) | Multiphase, axial, T1-weighted, 3D spoiled gradient-recalled echo with fat-saturation (breath held) |
| Axial, T2-weighted, fast spin echo with fat saturation (respiratory-triggered or navigator-gated or PROPELLER/BLADE technique) | Axial and coronal T1-weighted, 3D spoiled gradient-recalled echo with fat-saturation – 10- and 20-min delay (breath held) |
| Axial, T1-weighted, gradient-recalled echo (in and out of phase) (breath held) | — |
| Axial, T1-weighted, 3D spoiled gradient-recalled echo with fat saturation (breath held) | — |
| Axial, diffusion-weighted echo planar imaging (respiratory triggered or free breathing) | — |
a Can be power injected at 1 to 2 mL/s at 0.025 mmol/kg through 22-g peripheral intravenous cannula (or larger).
Benign pediatric liver lesions
Infantile Hemangiomas
Infantile hemangiomas are the most common tumor of infancy, occurring in up to 10% of infants. Recent data suggest that they are comprised of primitive blood vessels derived from angioblasts or placental stem cells that may implant on the fetus in utero. Infantile hemangiomas are most common in premature female Caucasian infants, those with a low birth weight, and those whose gestation was complicated by placental disruption or chorionic villous sampling. Although they can occur throughout the body, infantile hemangiomas are most frequently cutaneous, with the liver being the most common extracutaneous site. Hepatic lesions are seen in up to 13% of children with skin lesions.
Recent histopathological discoveries have caused vascular anomalies in children, including infantile hemangiomas, to be reclassified according to a biologic classification system first proposed in 1982. In this system, lesions are divided into 2 basic categories: neoplasms (masses that proliferate and grow by undergoing mitosis) and vascular malformations (congenitally deformed vessels that do NOT undergo mitosis). Infantile hemangiomas are true neoplasms that undergo mitosis during their proliferative phase, followed by gradual involution. They have been found to contain a unique pathologic marker, glucose transporter protein isoform 1 (GLUT1), which is not found in any other tissue, except for the human placenta. This system was accepted by the International Society for the Study of Vascular Anomalies (ISSVA) in 1996 and is now widely used among pediatric subspecialists. The utility of the ISSVA system lies in its ability to provide a systematic approach to vascular lesions that predictably correlates with history, lesion clinical course, imaging findings, accurate diagnosis, and treatment options. Hassanein and colleagues showed that 69% of children seen in a large vascular malformations clinic were initially given a wrong diagnosis leading to initial improper treatment in 20.6% of children. Hepatic hemangiomas are often further descriptively classified into 3 subtypes: focal, multifocal, and diffuse. Other terms used include hemangiomatosis (indicating multiple hemangiomas) and disseminated hemangiomatosis (applied when hemangiomas occur in 3 or more organ systems).
Infantile hemangiomas generally present between 2 weeks and 2 months of age with one or more skin or hepatic lesions. Lesions follow a predictable clinical course of initial proliferation over the first year of life followed by gradual involution that may extend into puberty. Although many children with infantile hemangiomas of the liver likely go undetected, these lesions may rarely come to attention because of a variety of associated complications, including high-output congestive heart failure, hypothyroidism, thrombocytopenia, hepatic failure, and respiratory distress caused by massive hepatomegaly. The primary differential diagnosis of hepatic hemangioma is metastatic neuroblastoma, which can be easily excluded in most cases with urine catecholamine screening and abdominal US to assess for a primary adrenal or paraspinous mass.
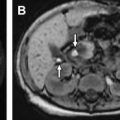
Although imaging of hepatic hemangiomas typically begins with sonography ( Fig. 1 ), these lesions generally require additional cross-sectional imaging (preferably MR imaging), and rarely biopsy, for definitive diagnosis. Infantile hemangiomas can demonstrate variable imaging features depending on their stage of development. Proliferative hemangiomas are generally well-defined, hypervascular, vigorously enhancing masses with internal flow voids. Multifocal lesions are usually small and uniform, whereas larger single lesions may contain central hemorrhage, necrosis, fibrosis, and, very rarely, calcifications. Classically, these lesions are T1-weighted hypointense, T2-weighted hyperintense, and demonstrate postcontrast hyperenhancement (see Fig. 1 ). Postcontrast T1-weighted images usually show early peripheral enhancement (either continuous or discontinuous), with variable subsequent centripetal filling in more delayed venous phases. This same pattern of enhancement has been described in adult hepatic lesions also known as hemangiomas (non-neoplastic GLUT1 negative vascular malformations). In infants, who often have numerous small hepatic hemangiomas, the pattern of centripetal enhancement is much less common and is not a reliable sign for diagnosis. These lesions often flash fill, that is, they appear homogeneously hyperintense during the arterial-phase postcontrast imaging ( Fig. 2 ). If a hepatocyte-specific contrast agent (HSA) is used, infantile hemangiomas vary in signal compared with the surrounding liver on the hepatocyte phase. As lesions involute, which occurs after the first year of life, hepatic hemangiomas show progressively less enhancement.