Introduction
The advancement of magnetic resonance imaging (MRI) has made high-quality visualization of the normal and pathologic spine, as well as associated neural tissue, readily available. Relative to plain radiography and computed tomography (CT), MRI boasts excellent visualization of soft tissues without exposing the patient to harmful radiation. Within the cervical spine specifically, MRI is routinely used for evaluation of neck pain and radicular symptoms. Several pathologies including spinal stenosis, degenerative disc disease, and tumors can be identified on MRI. However, as with any imaging modality, MRI also has the potential to highlight clinically insignificant pathologies, thus correlation with clinical exam findings is critically important to confirm a diagnosis. Regardless, high-quality visualization of anatomic structures and their associated soft tissues makes MRI a highly valuable clinical and diagnostic tool.
MRI Principles and Image Sequences of the Subaxial Cervical Spine
When clinical pathology is suspected in the cervical spine, classic radiographs are often the first imaging study to be performed. However, the inability to visualize soft tissues in detail leaves potential for certain diseases to be overlooked. Conversely, MRI is a noninvasive technique offering excellent soft tissue resolution. Because it does not involve the use of harmful ionizing radiation, MRI has quickly become the imaging modality of choice for many patients. Several MRI sequences exist to image the cervical spine in multiple planes, including T1-weighted spin echo (SE), T2-weighted fast spin echo (FSE), axial gradient-recalled echo (GRE), and more ( Table 1 ). Each sequence is uniquely different to highlight different elements of cervical anatomy and physiology, therefore appropriate sequence selection is imperative to diagnostic evaluation.
| MRI Sequence | Clinical Context | Strengths | Weaknesses |
|---|---|---|---|
| T1-weighted spin echo |
|
|
|
| T1‑gadolinium contrast-enhanced |
|
|
|
| T2-weighted fast spin echo |
|
|
|
| Gradient-recalled echo |
|
|
|
| Short tau inversion recovery |
|
|
|
| Fluid-attenuated inversion recovery |
|
| |
| Metal artifact reduction sequences |
|
| |
| Kinetic MRI |
|
| |
| Susceptibility-weighted |
|
|
With good image resolution and signal-to-noise ratios, T1-weighted SE sequences are best used to evaluate cervical anatomy, fracture lines, and osseous details. Superior depiction of bone marrow intensity also makes T1-weighted imaging a useful tool in identifying processes of bone marrow replacement, such as metastatic disease. However, T1-weighted imaging is less sensitive to many pathologies requiring clear differentiation of tissues. For example, poor distinction between intervertebral discs and the posterior longitudinal ligament or between muscle and nonfatty structures makes adequate assessment of these structures potentially difficult.
T1‑gadolinium contrast-enhanced (T1-Gd) sequences may be considered for detection of necrosis or inflammatory conditions. Increased blood vessel permeability secondary to disease permits accumulation of contrast agent which appears hyperintense to surrounding tissues. However, the use of contrast makes these sequences relatively invasive, time-consuming, and costly to perform.
T2-weighted sequences are produced using longer echo time (TE) and repetition time (TR) to improve visualization of pathologic changes in soft tissues (i.e., intervertebral discs), evaluation of cellular processes altering local water content, and assessment of spinal cord parenchyma for lesions and edema. In contrast to T1-weighted imaging, the spinal cord appears with intermediate signal intensity surrounded by high-intensity cerebrospinal fluid (CSF) on T2-weighted sequences, enabling clear identification of any areas of compromised central canal integrity. Specifically, T2-weighted FSE sequences have largely replaced SE sequences due to their faster imaging speed, improved resolution, superior signal-to-noise ratio, and decreased motion artifact. Disadvantages of T2-weighted FSE sequences include a decreased ability to detect small areas of calcification or hemorrhage, for which susceptibility-weighted MRI (SW-MRI) and T2-weighted GRE sequences provide better differentiation. Additionally, T2-weighted FSE may exhibit overly bright appearances of fat and CSF. Short tau inversion recovery (STIR) and fluid-attenuated inversion recovery (FLAIR) sequences are more appropriate for appreciating these tissues, respectively.
Although both T1-weighted SE and T2-weighted FSE sequences are good at detecting degenerative changes, each has its disadvantages. Neither clearly shows ossification of ligaments, so CT is the imaging study of choice should there be concern for this pathology. Further, fat can pose interference within these sequences, particularly in T1-weighted images. Fat-suppression sequences may therefore be necessary to achieve adequate contrast enhancement within the anatomic area of interest. Artifact, such as CSF pulsation on T2-weighted imaging or esophageal swallowing motions, may be present and should not be mistaken for pathology. Lastly, as with all MRI techniques, it is important to recognize that significant disease may be harbored within the areas between imaging planes on sequences. Gap size may need to be decreased in some cases to visualize tissues with more precise depth.
Should T1-weighted SE and T2-weighted FSE sequences produce inadequate images, axial GRE sequences can be ordered to supplement. Decreased imaging time and susceptibility to motion artifact establish GRE as superior in detecting many degenerative changes such as osteophyte formation and neural foraminal narrowing. Additionally, this sequence is also useful in detecting the presence of blood following spinal cord hemorrhage in the context of trauma or vascular malformation. While GRE demonstrates good sensitivity for extradural disease processes, its sensitivity for intradural pathology is less impressive.
Increasing in popularity are inversion recovery sequences such as STIR and FLAIR. With their reduced scan time and low susceptibility to metal, these sequences are ideal for imaging patients with orthopedic hardware. As mentioned previously, STIR is a fat-suppression sequence that proves useful when adipose tissue is obstructing the images. STIR can be added onto T1- or T2-weighted sequences, but it cannot be used after a gadolinium injection due to inadvertent nulling of certain tissues. Due to its superior sensitivity for detecting edema, STIR sequences are commonly ordered for the evaluation of traumatic injury. FLAIR sequences may be added onto T2-weighted scans for suppression of motion artifact caused by water, making them a preferred sequence for pediatric patients or those who cannot remain supine for extended periods of time. By attenuating the high-intensity signal of CSF, these sequences can better distinguish the spinal cord, CSF, and intervertebral disc for evaluation of degenerative changes. FLAIR further serves as a valuable tool in assessment of demyelinating disease, such as multiple sclerosis.
Artifact reduction is also achievable using metal artifact reduction sequences (MARS). These sequences are particularly useful for imaging the cervical spine in patients with metal implants, as use of a low-powered magnet reduces the size and intensity of metal artifact produced by magnetic field distortion without sacrificing signal-to-noise ratio.
Kinetic MRIs, such as flexion/extension sequences, are newly emerging techniques used to assess the effects of movement and gravity on the cervical spine. Utilizing these methods may allow physicians to unveil motion-dependent pathologies not seen in more classic imaging orientations. Several pathologies can be exacerbated with movement and should be evaluated using kinetic MRI, including central spinal stenosis, spondylolisthesis, and rheumatoid arthritis (RA). Furthermore, cervical alignment may also be better assessed with these techniques, as the effects of gravity contribute to a more accurate clinical evaluation of spinal curvature.
Susceptibility-weighted MRI (SW-MRI) is an emerging MR technique that has demonstrated improved visualization of calcified structures, such as osteophytes. SW-MRI has high sensitivity and specificity for identifying and locating osteophytes relative to classic T1- and T2- weighted images, which often fail to distinguish osteophytes from disc herniations. Whereas T1- and T2-weighted sequences show both as hypointense, SW-MRI depicts calcified structures with a hyperintense signal, a useful benefit for preoperative planning not available with conventional 2D radiography.
Anatomy
Vertebral Anatomy
The cervical spine consists of seven vertebrae arranged vertically from the base of the skull to the level of the shoulders. In coordination with associated muscles, tendons, and ligaments, these bony structures provide structure, support, and flexibility to the neck while serving to protect the spinal cord coursing within. Generally, the cervical spine shares characteristics with other spinal segments, including a vertebral body, vertebral arches, transverse processes, a spinous process, and facet joints. In midsagittal view, MRI of the healthy cervical spine shows small lips protruding from the anteroinferior border of the vertebral bodies, facilitating a normal lordotic curvature from C2-T2 with the apex around C4-C5. In contrast, the superior faces appear concave in the lateral direction and convex anteroposteriorly, and the vertebral body walls are slightly concave in nature. The fatty composition of the marrow within the healthy vertebral body conveys a bright signal intensity to T1-weighted images. Moving parasagittally brings the transverse processes into frame, which serve as significant points of attachment for various muscles and ligaments and provide conduits for neurovasculature. The vertebral artery and venous systems can be seen in the axial perspective passing vertically through the foramen transversarium of the transverse processes on each side of the vertebra. Notably, the spinous processes of the typical cervical vertebrae demonstrate a classically bifid structure. Representative MRI images of the normal cervical spine are shown in Fig. 1 .

There are a few exceptions to these anatomic generalizations within the cervical spine. C1, often referred to as the atlas, is unique in its replacement of a vertebral body with the anterior tubercle. As the only vertebra without a vertebral body, the atlas assumes a ring-like shape and connects the occipital condyle above with the proper cervical spine below via the atlanto-occipital joint. Extensions of the anterior longitudinal ligament and ligamentum flavum comprise the atlanto-occipital membranes, which support the atlanto-occipital joint in conferring a majority of the flexion/extension motion to the head. Inferior to the atlas is the C2, or axis, so named due to the bony odontoid process protruding upward into the ring of C1 with which it forms the atlanto-axial joint. This synovial joint permits the atlas to rotate around the axis and accounts for nearly 50% of the rotational motion of the head. Because they exhibit such unique morphologies, C1 and C2 are classified as atypical vertebrae. Additionally, the most inferior vertebra of the cervical spine, C7, has a number of distinguishing features. Known as vertebra prominens, C7 can be identified on midsagittal view by its particularly prominent spinous process, which is not bifid as in the rest of the cervical spine. This specialized projection ensures optimum fit with T1 in the cervicothoracic junction and provides attachment points for more muscles than any other cervical vertebra. Furthermore, the foramina in the transverse processes where the vertebral arteries normally pass are absent at this level and will not be seen on axial view.
Facet Joints
At each vertebral level except C1, the laminae and pedicles merge laterally into lateral masses before separating into the paired superior and inferior articular processes. The inferior processes from the vertebra above articulate with the superior processes of the vertebra below to form the facet joints. Formally termed zygapophyseal joints, these true synovial joints contain cartilage and menisci to permit small movements across the motion segment while restricting hyperflexion and hyperextension. Movement across each motion segment is quite limited; however, the cumulative effect across the entire spine enables significant rotation, flexion, extension, and lateral bending. Notably, whereas these joints are oriented vertically within the lumbar spine, their 45-degree orientation within the cervical spine facilitates flexion and extension. Paired with the abundance of proprioceptive and pain receptors innervating the area, the susceptibility of the facet joints to age- or trauma-related degeneration denotes a plausible role for the facet joints in chronic neck pain. Visualization of the facet joints is best achieved with T2-weighted imaging, which shows a smooth homogenous signal of the hyaline cartilage overlying the low-signal cortical bone. Despite the paucity of literature surrounding visualization of the normal facet joints with MRI, this imaging method becomes increasingly important in the context of pathology such as joint inflammation and age-related cartilage degeneration.
Intervertebral Discs
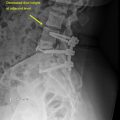
With the exception of the atlanto-occipital and atlanto- axial joints, intervertebral discs are interspersed between adjacent vertebral bodies throughout the cervical spine. These avascular structures provide support to vertebral bodies, permit flexibility throughout the full range of head and neck movements, and cushion the impact of various stresses and loads on the cervical spine. The anatomy of the intervertebral disc consists of two basic components. The nucleus pulposus is the soft inner core of mucoprotein gel that assists in providing cushioning and flexibility to the disc. Surrounding the nucleus pulposus is the annulus fibrosus, a durable external layer composed of concentric rings of alternating obliquely oriented collagen fibers functioning to distribute forces, protect the inner core, and stabilize the motion segment. MRI is used to assess the integrity of the intervertebral discs through quantification of disc hydration primarily based on proton density and water content. Specifically, normal discs exhibit a bright signal within the nucleus pulposus on both T1- and T2-weighted images due to its high water and proteoglycan content, often with a horizontally oriented area of decreased intensity at the core’s center in the adult. The more fibrous composition of the annulus fibrosus emits a darker signal, generating a marked distinction between the nucleus pulposus and annulus fibrosus in the healthy disc ( Fig. 1 C). Coronal views show the superior face of the disc is concave, while the inferior face is convex. In midsagittal view, one can determine that the six intervertebral discs of the cervical spine are thinner than the greater load-bearing discs of the lumbar spine but thicker than those of the less-mobile thoracic region. The discs of the healthy cervical spine also demonstrate greater thickness anteriorly, which in conjunction with the lipped nature of the vertebral bodies facilitates a lordotic curvature. Normally, an intervertebral disc will be entirely contained within the anterior and posterior margins of the vertebral bodies and will not vary in height by more than 25% compared to adjacent normal disc spaces.
Vertebral endplates mark the transition between the intervertebral disc and the adjacent vertebral body. Without a blood supply of its own, the intervertebral discs rely on the transport of nutrients and small amounts of blood from the subchondral bone through the hyaline cartilage and porous bone of the vertebral endplate for nourishment. These structures also function to buffer disc pressures on the vertebral bodies and limit protrusion, provide points of insertion for the inner fibers of the annulus, and serve as growth plates responsible for endochondral ossification. On T1-weighted MRI, normal endplates display uniform intensity with the vertebral body and a clear demarcation from the adjacent intervertebral disc, whereas alterations in signal intensity relative to the vertebral body indicate endplate abnormalities ( Fig. 1 C).
Spinal Canal
One of the most critical functions of the vertebral column is to house and protect the spinal cord within a spinal canal created by stacked vertebral foramen. The spinal cord itself is immediately surrounded by the pia mater, subarachnoid space, arachnoid mater, and dura mater. At the level of each intervertebral disc, bilateral spinal nerves project off of the spinal cord to innervate their respective body segments. The entirety of the cervical spinal cord is best visualized in midsagittal view. On T1-weighted images, the spinal cord will appear with an intermediate signal due to abundance of lipids within the cord. However, due to low water content of the cord, this structure emits low-intensity on T2-weighted images.
Despite the paucity of MR-based studies describing the normal configuration of the cervical canal, dural tube, and cord, a few generalizations can be made. From the axial and sagittal perspectives of T2-weighted sequences, the healthy spinal cord will show a homogeneous signal without intrinsic abnormalities. Typically measured on T2-weighted images, canal size varies by vertebral level, as well as by the sex and age of the patient. Canal diameter normally tapers from the first vertebrae to the third vertebrae, where the spinal cord undergoes a slight enlargement before retaining a more uniform diameter throughout the remainder of the cervical spine. Notably, cross-sectional area, sagittal diameter, and axial diameter of the spinal cord peak in the third decade of life and tends to decrease thereafter ( Table 2 ).
| Age | Men | Women |
|---|---|---|
| 20–29 | 12.7–14.4 mm | 12.6–14.3 mm |
| 70–79 | 11.0–13.6 mm | 10.8–13.5 mm |
Vertebral Column Alignment
In sagittal view, the entirety of the vertebral column assumes a slightly S-shaped appearance to efficiently perform the necessary functions of each spinal region ( Fig. 1 A). Generally, the curves of the spinal column serve to absorb shock, provide balance, and permit motion. To aid in supporting the weight of the head, the cervical spine maintains a lordotic curvature that should be apparent in sagittal view of any radiograph or MRI sequence. The extent of lordosis is best measured on upright/standing X-ray, as conventional MRI is performed in the supine position where gravity can alter the patient’s typical alignment. However, if upright/standing films are not available, methods do exist to estimate the extent of lordosis on MRI (in supine position), such as Cobb angles. However, the translation of these measurements to MRI sequences has been only scarcely reported in literature and their validity is yet to be established.
Soft Tissues
The soft tissue of the cervical spine refers to the muscles, ligaments, fascia, blood vessels, nerves, and lymphatics that traverse and supply structures of the region.
Muscle
Musculature accounts for the majority of soft tissue volume and contributes to movement of the head, neck, upper back, and shoulders. At the dorsolateral aspect of the cervical spine from superficial to deep are the trapezius, splenius capitis, splenius cervicis, levator scapulae, rotatores, rectus capitis, obliquus capitis, and semispinalis muscles. Anterolaterally are the platysma, sternocleidomastoid, strap muscles of the larynx, hyoid muscles, scalenes, longus cervicis, and longus colli muscles. The bodies of these muscles are illustrated well on nonfat-saturated T1-weighted MRI, while T2-weighted imaging allows assessment of edema and fluid.
Ligaments
Muscles are assisted in providing stability to the cervical spine by a network of ligaments, including the anterior longitudinal ligament (ALL), posterior longitudinal ligament (PLL), and posterior ligamentous complex (PLC). The ALL and PLL course along the anterior and posterior aspects of the subaxial vertebral bodies, respectively, demonstrating a narrow, thick morphology over the bodies but stretching wide and thin while coursing over the intervertebral discs. The PLC is located posterior to the neural arch, consisting of the ligamentum flavum (LF), interspinous ligaments, and ligamentum nuchae. Within the cervical spine, the interspinous ligaments are particularly thin and poorly developed. Due to their fibrous composition, ligaments appear as dark bands on all MRI pulse sequences, with discontinuities in signal void suggesting acute ligament rupture.
Vasculature
Structures of the cervical spine are supplied by branches of the vertebral, ascending cervical, deep cervical, and occipital arteries. The vertebral arteries originate from the subclavian arteries, coursing between the longus colli and anterior scalene before entering the vertebral column at the level of C6 and ascending through the transverse foramina toward the foramen magnum. Anterior and posterior vertebral veins accompany the vertebral arteries, surrounding them in a venous plexus at the lateral aspect of the spine. Upon entering the spinal canal, the left and right vertebral arteries merge to form the basilar artery that supplies the brainstem. Several segmental radicular arteries stem from the vertebral arteries, mostly directed toward the ALL, intervertebral disc, and vertebral body. Others feed into the muscles of the neck, where they anastomose with branches of the ascending cervical artery traveling between the anterior scalene and longus capitis along the transverse processes. The deep cervical artery arises from the costovertebral trunk and travels between the facet joints toward the posterior spinal muscles, while the occipital artery stems from the external carotid artery on its way to supply the posterior scalp. Conventional MRI can be used to evaluate vasculature in the cervical spine. Because MRI cannot recognize flow, however, vasculature is illustrated as focal areas of absent signal, or “flow voids” ( Fig. 1 B). Normal vasculature should exhibit a single lumen and generally follow the course outlined above. Administration of contrast dye in magnetic resonance angiography (MRA) enables enhanced visualization of these structures and is the preferred method for their assessment.
Nerves
Assessment of the nerves and the foramina through which they pass is a critical component MR-based evaluation of the cervical spine. On midsagittal view, the spinal cord can be appreciated extending inferiorly from the medulla oblongata and pons. At each vertebral level, branches stem from the spinal cord to form the eight spinal nerves of the cervical spine, termed C1 through C8. These then travel along the costotransverse lamella and between the anterior and posterior tubercles of the transverse processes before exiting the vertebral column via the intervertebral foramina toward their respective dermatomes and myotomes. Parasagittal views depict normal spinal nerves with intermediate intensity surrounded by high-intensity fat on T1-weighted imaging. Because of the 45-degree oblique orientation of the intervertebral foramina, however, oblique imaging offers optimal viewing to assess whether compression or irritation may be contributing to any pain or dysfunction.
Pathologies of the Cervical Spine
Central Spinal Stenosis
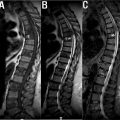
Central spinal stenosis encompasses a spectrum of structural changes within the cervical spine causing narrowing of the canal. This disease can result from several spinal abnormalities and often manifests clinically with neck pain and/or upper extremity radiculopathy. Stenosis severe enough to cause impingement and injury to the spinal cord is referred to as myelopathy, a disease predominantly associated with aging populations and most commonly diagnosed in patients over 50. With its clear depiction of the spinal cord and avoidance of the magnification errors that commonly affect radiographs, MRI is an essential tool for evaluating for spinal stenosis. Furthermore, MRI offers superior visualization of the intervertebral discs, osteophytes, ligaments, and degree of stenosis for superior determination of potential causes and severity of disease. Because cervical spinal stenosis can vary with dynamic change, often worsening upon extension, kinetic MRI sequences may be considered. These sequences may prove useful when selecting a level for cervical decompression during preoperative planning. Generally, patients with suspected cervical myelopathy should undergo MRI to confirm the diagnosis before considering surgical intervention. If confirmed, treatment should be pursued promptly as prolonged spinal cord compression can permanently damage the structure, resulting in residual symptoms and functional disabilities. Studies have shown treatment initiated within 6 months of symptom onset confer the greatest chance of recovery.
At the level of stenosis, intramedullary signal changes can be seen within the spinal cord on T1- and T2-weighted images. T2-weighted MRIs will additionally show CSF as hyperintense signal both anterior and posterior to the cord on midsagittal images, as well as circumferentially around the cord on axial images, making physical compromise of the cord easily distinguishable regardless of compression mechanism ( Fig. 2 ). Disease progression may bring an increase in spinal cord edema, increasing T2-weighted and potentially decreasing T1-weighted signal intensity. Signal hyperintensity on T2-weighted sagittal images is often considered to be a diagnostic marker of myelopathy in the cervical spine, but the signal change is not pathognomonic for myelopathy. Axial imaging is seldom used to assess for myelopathy as its validity remains uncertain. However, a “snake eye” appearance on T1-weighted axial scans has been noted in literature to be associated. T1-Gd studies may be necessary to rule out vascular, inflammatory, infective, neoplastic, and demyelinating processes that may present similarly to myelopathy on conventional MRI sequences.

C5-C6 is often the level most severely affected by myelopathy, followed by C4-C5 Central canal size varies among individuals; therefore, it is crucial to evaluate the affected level in reference to adjacent levels when assessing for stenosis and associated myelopathy. Nevertheless, an inverse relationship between canal size and cord compression has been thoroughly described throughout the literature. Reliable methods have been developed for quantification of central spinal stenosis severity, regardless of etiology. For example, Muhle et al. and Kang et al. have each suggested grading systems, as described later in this chapter.
Surgical intervention is commonly pursued in patients who exhibit clinical symptoms and display evidence of compression that could cause radiculopathy, myelopathy, or a combination of the two on MRI. For many, spinal cord decompression is an effective treatment for alleviation of symptoms and prevention of disease progression. Specifically, patients with hyperintense intramedullary signal change on T2-weighted images are more likely to experience good surgical outcomes and reversal of MRI abnormality, while those with low intramedullary signal on T1-weighted scans may experience less favorable outcomes. The limited regenerative capacity of the spinal cord should always be appreciated during spinal surgery, requiring adequate precautions and proper technique throughout the perioperative period to minimize risk of permanent injury.
Certain risk factors for spinal stenosis should be discussed with patients if identified on imaging. For example, congenital narrowing of the cervical spinal canal less than 13 mm in diameter is associated with increased the risk of developing cervical spinal stenosis. Specifically, a congenital canal diameter of less than 13 mm is diagnosed as relative stenosis, whereas a diameter of less than 10 mm is classified as absolute stenosis. More recently, Nouri et al. suggested a SCOR measurement of greater than 70% is diagnostic for congenital spinal stenosis. These patients are more likely to develop myelopathy at a younger age with a greater degree of impairment and disability. However, these patients demonstrate comparable surgical outcomes to patients without congenital spinal stenosis.
Degenerative Disc Disease
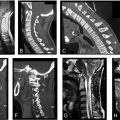
Degeneration within the cervical spine can affect many different structures, including the vertebral bodies, spinal ligaments, and facet joints. However, initial stages of degenerative change typically present as a loss of structural integrity of the intervertebral discs, known as degenerative disc disease (DDD). Symptoms associated with DDD can present at any age, often with gradual-onset chronic pain. Progressive disc degeneration can lead to unequal exertion of forces on the adjacent vertebral bodies, resulting in bone restructuring and/or osteophyte formation characteristic of spondylosis. On MRI, a healthy nucleus pulposus will appear hyperintense on T1- and T2-weighted images, but it loses intensity with age as water and proteoglycan content decreases. This normal physiologic process predisposes the discs to further degradation, leading to diminished disc height, annular tears, disc herniation, and autofusion of adjacent vertebral bodies in the event of complete collapse.
T1- and T2-weighted sequences are considered to be the most sensitive imaging methods for evaluating the intervertebral discs ( Fig. 3 ). However, MRI often fails to distinguish clinically relevant findings from incidental ones. This can be disadvantageous in the context of DDD as signs apparent on imaging do not always correlate with symptoms. Furthermore, it is important to recognize that decreased T2-weighted signal intensity in the nucleus pulposus due to loss of water and proteoglycan can be detected by as early as the second decade of life, as the core undergoes transitions to a more fibrocartilaginous structure and the distinct border between nucleus pulposus and annulus fibrosus becomes attenuated. Loss of clear demarcation between these structures is not considered significant unless acute injury is suspected. Several additional signs of DDD are apparent on MRI. Loss of disc height can be determined by comparison to normal values or to normal adjacent disc heights, with decreases of greater than 25% serving as the diagnostic threshold for degeneration. More progressive degeneration characterized by accumulation of nitrogen within the disc results in the vacuum phenomenon, appearing as signal void on both T1- and T2-weighted imaging. These degenerative changes can be more specifically diagnosed using any of the grading systems described below, such as Pfirrmann grades.

Herniated Disc
A common and severe complication of degenerative disc disease is herniation of the intervertebral disc, a pathology in which a fragment of the nucleus pulposus extrudes through a tear or rupture in the annulus fibrosus. As DDD progresses, nucleus pulposus contents may be forced to migrate anteriorly, posteriorly, or laterally. Posterior herniations can project beyond the vertebral body margin to potentially cause cord compression, myelopathy, and radicular symptoms. Disc herniations posterolaterally are particularly likely to impinge on spinal nerves exiting the vertebral column to produce clinically relevant symptoms, while anterior herniations have been reported to cause dysphagia by compressing the esophagus.
MRI is considered the superior imaging method for evaluation of disc herniation. Herniated discs are most commonly found at the levels of C5-C6 and C6-C7, causing impingement of the C6 and C7 nerve roots, respectively. Conversely, impingement of the C3 nerve root by a herniated disc is rare. On T2-weighted images, disc herniations are appreciable on sagittal and axial scans as hypointense protrusions of the disc into the vertebral foramen ( Fig. 4 ). Cord compression is best visualized from the sagittal perspective as a hypointense herniation impinging on the hyperintense spinal cord. Disc herniation is also commonly associated with Modic changes within the vertebral endplates superior and inferior to the disc, described later in this chapter. Clinically, it is not possible to determine the age of the disc herniation unless the disc appears well hydrated without any osseous ridging, suggesting the herniation is acute or subacute in nature. Chronic herniations secondary to DDD are more common, however.

Characteristic of all herniated intervertebral discs is the presence of high-intensity zones (HIZs) within the affected disc. HIZs are focal areas of high intensity often within the posterior annulus fibrosus seen in sagittal view on T2-weighted imaging ( Fig. 5 ). Notably, HIZs do not result from normal aging, making them a reliable marker of DDD when differentiating from pathologically aging discs. Appearing most commonly in the posterior aspect of the annulus fibrosus, HIZs are produced by a protrusion of the hyperintense nucleus pulposus through a tear in the annulus. These findings are highly correlated with significant lumbar discogenic pain, though their clinical relevance in the cervical spine remains to be elucidated.

Spondylolisthesis
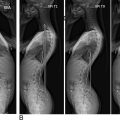
Spondylolisthesis is a relatively common condition resulting from degeneration of the facet joints or hypermobility syndromes. On imaging, spondylolisthesis will appear as a horizontal translation of one vertebral body out of alignment with adjacent vertebral bodies in sagittal view ( Fig. 6 ). This disease is often associated with significant degeneration, disc herniation, and instability of the cervical spine. With significant displacement, the diameter of the spinal canal can become compromised to produce spinal stenosis. Anterolisthesis, or forward slippage of the vertebral body, is commonly associated with more severe degeneration or trauma, though RA and other inflammatory arthropathies are known correlates of atraumatic spondylolisthesis.

Since many spondylolistheses in the cervical spine are dependent upon normal upright positioning, diagnoses are commonly overlooked on conventional supine MRIs. Additionally, movement-dependent spondylolistheses may not be apparent on static MRI, requiring flexion/extension kinematic sequences. The current gold standard method for evaluating spondylolisthesis is the Meyerding classification system using upright radiographs, though few studies have examined the validity of this system with MRI. More recently, however, Kawasaki et al. described a method for measuring severity of spondylolisthesis that is performed on upright kinetic MRI (see below).
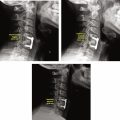
Osteoarthritis of the Facet Joints
Osteoarthritis of the facet joints may develop independently but most often occurs secondary to degeneration of the intervertebral disc with loss of disc space height. As in any true synovial joint, degeneration of the facet joint is marked by fibrillation, fissuring, and ulceration of the hyaline articular cartilage advancing from superficial to deeper layers. Although less accurate in visualizing bony pathology, T2-weighted MRI demonstrates moderate-to-good correlation with CT in identifying osteoarthritis and offers enhanced definition of nonbony structures in multiple planes, making it an acceptable substitute for assessing facet joint morphology ( Fig. 7 ). In early stages of the disease, cartilage degeneration causing narrowing of the joint space can be detected on axial view as decreased distance between the superior and inferior articulating facets. As the disease progresses, progressive decrease in joint space is accompanied by facet bone proliferation, forming osteophytes that appear isointense to adjacent bone. Subchondral sclerosis may also develop, emitting hypointense signal on both T1- and T2-weighted images. In contrast, formation of cysts in the subchondral bone generates hyperintense T2-weighted signal if there is direct communication with the facet joint and hyperintense T1-weighted signal if there is a hemorrhagic or proteinaceous component, as seen from the axial or sagittal perspective. Joint hypertrophy denotes later stages of facet joint osteoarthritis and is better appreciated on CT. With the potential to narrow the central canal, lateral recesses, and foramina, facet joint osteoarthritis has significant implications in the etiology of neck and back pain.

Osteophytes
Osteophytes are projections of bone resulting from abnormal osteogenic proliferation commonly seen in the degenerative cervical spine at points experiencing chronic strain. In the cervical spine specifically, osteophyte development is commonly a response to inhomogeneity in pressure on the vertebral endplate and facet joint secondary to intervertebral disc degeneration. Three types of osteophytes have been described according to their shape and location ( Fig. 8 ). Traction osteophytes refer to 2–3-mm bony structures projecting horizontally from the vertebral endplate. Claw osteophytes also project from the endplate, arching over the disc toward the adjacent vertebral body to form a claw shape. The third type, known as wraparound bumper osteophytes, may be found along the capsular insertion of the facet joints and are commonly seen in progressive facet joint osteoarthritis. MRI can be used to identify the presence of osteophytes; however, they can be difficult to distinguish from disc herniations as both will appear hypointense on T1- and T2-weighted imaging. SW-MRI can be used for improved differentiation of these structures, conveying a hyperintense signal to calcified structures such as osteophytes on inverse magnitude and phase-filtered images. Another benefit of SW-MRI is its ability to determine the dimensions and precise location of osteophytes, which is difficult to achieve on 2D radiography and useful in preoperative planning.

Cervical Injury
Imaging of the cervical spine after trauma is essential for proper identification and classification of injury morphology. The development of the subaxial cervical spine injury classification system (SLIC) has sought to normalize assessment by dividing spinal injuries into three categories: compression, distraction, and translation/rotation ( Table 3 ). Compression injuries include those causing loss of height in part or all of the vertebral body and/or disruption of the vertebral endplate, including compression fractures, burst fractures, sagittal or coronal plane fractures, and flexion compression fractures. Trauma causing vertical dissociation of the vertebral column are labeled as distraction injuries and usually involve disruption of the ligaments at the level of the disc space or facet joints. Hyperextension injuries to the ALL resulting in either anterior disc expansion or posterior compression causing posterior element fracture or spinal cord compression are included in this category. Translational/rotational injuries refer to those demonstrating greater than 11 degrees of horizontal displacement of one vertebral segment relative to another. Trauma of this nature typically includes fracture or dislocation of the facet joints, lateral mass separation, or bilateral pedicle fractures. The integrity of the discoligamentous complex (DLC), consisting of the intervertebral discs, ALL, PLL, ligamentum flavum, interspinous ligaments, supraspinous ligaments, and facet capsules, is another component of the SLIC as these structures directly contribute to stability of the cervical spine. Widening of the space between adjacent spinous processes, dislocation (< 50% articular apposition) or separation of the facet joints, subluxation of the vertebral bodies, and abnormal disc space widening are indicators of DLC compromise. The third and final component of SLIC is evaluation of neurologic status. As the spinal cord and nerve roots are normally protected within the spinal canal, neurologic impairment following traumatic injury is thus a logical indicator of severity of spinal cord injury.
| Injury Type | Morphology |
|---|---|
| Compression |
|
| Distraction |
|
| Translation/rotation |
|
MRI is regarded as the preferred imaging modality for evaluating patients with traumatic injury and neurologic compromise as it provides optimal assessment of soft tissue injury. Image acquisition performed within 72 h of injury prior to resorption of edema or extravasated blood is ideal, as the hypersensitivity of these fluids on T2-weighted imaging serves as an excellent natural contrast medium for ligament visualization. Comprehensive imaging should include T1-weighted, T2-FSE, gradient-echo, and FSE inversion recovery sequences in the sagittal plane as well as T2-weighted or gradient-echo sequences in the axial plane. Coronal T1-weighted or gradient-echo sequences may also be performed. The presence of fracture on MRI is determined by altered configuration of the vertebra or a break in cortical continuity with or without changes in signal intensity on T1- or T2-weighted scans. Edema of the marrow emits low intensity on T1-weighted and high intensity on T2-weighted imaging, while spinal cord edema shows intramedullary foci of T2-weighted hyperintensity. Intramedullary hematoma is indicated by foci of high signal intensity on T1-weighted and low intensity on T2-weighted, spin-echo, and gradient-echo sequences. Traumatic disc herniation is identified by disc protrusion with increased T2-weighted signal intensity in the disc tissue and associated paraspinal soft tissue or spinal cord damage. Whereas normal intact ligaments appear as dark bands on T2-weighted scans, high signal intensity in the ligament itself or clear interruption of the dark band suggests ligament injury. Pre- and paravertebral hemorrhage or edema can present with or without soft tissue swelling and presents as T1-hypointense and T2-hyperintense. Overall, MRI has been shown to be superior to CT in evaluation of traumatic and focal disc herniation, cord edema and compression, canal stenosis, ligament damage, and pre- and paravertebral hemorrhage and edema. However, appreciation of osseous injury such as degenerative subluxation, facet spondylosis, foraminal stenosis, and fracture is best achieved on CT.
Tumors
Metastatic tumors
Although less common than metastases to the thoracic and lumbar spine, metastatic tumors in the cervical spine are more common than primary bone sarcomas. The aggressiveness of the tumor, level within the spine location within the vertebra, and inclusion of associated anatomic structures each play a role in the clinical effects of the disease. Tumor growth requires the displacement or destruction of native bone tissue, compromising the integrity of the vertebrae and potentially leading to kyphotic deformities and compression of neurovasculature, causing neurologic dysfunction and ischemia. Metastatic disease most often affects the mid-cervical region (C3-C6), where a narrower spinal canal increases the risk of myelopathy, sagittal imbalance, and pain. At the level of the vertebra, the tumor usually begins at the posterior aspect of the vertebral body and advances anteriorly, destroying cortical bone within the pedicles, lateral masses, and transverse processes along the way. Diagnostic imaging of spinal neoplasia is best performed with MRI due to its superior visualization of soft tissue, assessment of edema, differentiation between normal and pathologic tissue, and identification of solid and cystic components. Although there are no specific features of metastatic disease on MRI, tumors and associated edema and syrinx generally emit intense signal on T2-weighted imaging.
Primary spinal cord tumors
Approximately half of spinal cord tumors are primary tumors, which are categorized into two types: intradural extramedullary spinal cord tumors (IESCTs) and intramedullary spinal cord tumors (ISCTs). IESCTs arise from within the spinal cord and account for 80% of primary spinal cord tumors. These include nerve sheath tumors (NSTs), such as schwannomas and neurofibromas arising from the perineural cells, and meningiomas originating from arachnoidal cells along the neuraxis. While each of these tumors has characteristic findings on MRI, extramedullary tumors generally show displacement and compression of the spinal cord, expansion of the thecal sac, and a distinct interface with surrounding cerebrospinal fluid. Most IESCTs are depicted with high intensity on T2-weighted and intermediate intensity on T1-weighted imaging. The remaining 20% of primary spinal cord tumors are the ISCTs, which originate from the dura and are located within the subarachnoid space. ISCTs typically present with reactive cysts and extensive edema, causing mild expansion of the spinal cord. The most common tumors in this category are ependymomas, astrocytomas, gangliomas, and hemangioblastomas, most of which appear as well-circumscribed masses emitting iso- or hypointense signal on T1-weighted and hyperintense signal on T2-weighted MRI.
Primary bony tumors
Tumors originating in bone may also develop within the cervical spine. Most primary bone tumors are benign, including aneurysmal bone cysts, giant cell tumors, osteoid osteomas, and osteoblastomas. These often mimic the general pattern of the spinal cord tumors on MRI: low to intermediate intensity on T1-weighted and high intensity on T2-weighted scans. Although much more rare, metastatic primary bone tumors carry a very poor prognosis as they are difficult to excise with wide margins. The most common primary metastatic bone tumors of the cervical spine are chordomas, osteosarcomas, chondrosarcomas, and Ewing sarcomas, each exhibiting a rather variable appearance on MRI based on subtype, progression, and extent of mineralization.
Spinal Deformity
Spinal deformity, such as scoliosis, hyperlordosis, and kyphosis, can lead to a plethora of secondary conditions with a variety of clinical presentations. MRI is seldom the imaging method of choice to evaluate vertebral alignment but is required to assess for infantile or juvenile idiopathic scoliosis, congenital bony abnormalities, and scoliosis when associated with neurologic symptoms. Should MRI be used or if upright radiographs are unavailable, vertebral alignment can be estimated by calculating the Cobb angle. However, the reliability and validity of this measuring technique for MRI have yet to be adequately established. Many patients with spinal deformities choose to undergo surgical therapy with implantation of hardware, potentially making postoperative imaging of the spine difficult. Subsequent imaging studies should employ metal artifact reduction MRI sequences for optimal visualization of structures. An example of MRI representing loss of cervical lordosis is shown in Fig. 9 .