Magnetic resonance (MR) neurography has progressed in the past 2 decades because of rapid technological developments in both hardware and software. In addition to improvements in high-resolution anatomic pulse sequences, functional techniques are becoming feasible. This article presents the current state-of-the-art three-dimensional anatomic techniques, discusses the advantages of functional techniques being exploited, and portrays novel contrast types and molecular techniques that are under development and promise a bright future for this rapidly evolving technique.
Key points
- •
Magnetic resonance (MR) neurography is an excellent technique for axial and multiplanar depiction of peripheral nerve anatomy and disorders.
- •
Three-dimensional isotropic spin-echo–type imaging is currently being used on high-field scanners for longitudinal demonstration of nerve disorders for the benefit of referring physicians.
- •
Whole-body MR imaging is being widely used to image tumors. Whole-body MR neurography holds promise in the depiction of diffuse peripheral nerve disorders and neurocutaneous syndromes.
- •
Diffusion-weighted imaging and diffusion tensor imaging permit functional imaging of nerves and related lesions, and allow tractography for presurgical planning and postsurgical follow-up.
- •
Magnetization transfer imaging and nerve-specific MR contrast agents are under development and in feasibility stages for the assessment of nerve degeneration and regeneration, which is beyond the scope of anatomic pulse sequences.
Introduction
Magnetic resonance (MR) neurography (MRN) is a noninvasive technique using high-resolution magnetic resonance (MR) imaging to diagnose peripheral nerve disorders and their underlying causes, such as indirect or direct penetrating injury, compression, stretch, friction, and iatrogenic insult, as well as to monitor processes of peripheral nerve degeneration and regeneration. At present, anatomic MRN is being widely used for a variety of nerve disorders. Because of the continuous technological advancements, MRN diagnostic capabilities have improved in the last 2 decades, and MRN is therefore likely to play an important role in the diagnostic algorithm of peripheral nerve disorders. This article reviews evolving novel MRN technologies currently used and under development with regard to their potential to meet the requirements for noninvasive imaging of peripheral nerves in both clinical and research settings.
Introduction
Magnetic resonance (MR) neurography (MRN) is a noninvasive technique using high-resolution magnetic resonance (MR) imaging to diagnose peripheral nerve disorders and their underlying causes, such as indirect or direct penetrating injury, compression, stretch, friction, and iatrogenic insult, as well as to monitor processes of peripheral nerve degeneration and regeneration. At present, anatomic MRN is being widely used for a variety of nerve disorders. Because of the continuous technological advancements, MRN diagnostic capabilities have improved in the last 2 decades, and MRN is therefore likely to play an important role in the diagnostic algorithm of peripheral nerve disorders. This article reviews evolving novel MRN technologies currently used and under development with regard to their potential to meet the requirements for noninvasive imaging of peripheral nerves in both clinical and research settings.
Nerve anatomy and peripheral neuropathy
To understand the new MRN technologies, as well as related normal and abnormal appearances of the peripheral nervous system (PNS) using these techniques, an understanding of the peripheral nerve structure, composition of its different tissues, and knowledge of the widely used classification of peripheral neuropathy is important.
The axon is the functional unit of the peripheral nerve, supported by surrounding Schwann cells and myelin layers. A layer of loose connective tissue, the endoneurium, surrounds each axon and its Schwann cells, to form a nerve fiber. Multiple nerve fibers are enclosed in robust connective tissue, the perineurium, to form a nerve fascicle. All nerve fascicles are surrounded by the epineurium, to form a peripheral nerve. Overall, peripheral nerve morphology shows a strong longitudinal order of its different compositional tissues. As a consequence, the axoplasmatic flow within peripheral nerves and, at a molecular level, the diffusion of water protons is aligned along these longitudinal structures.
Peripheral neuropathy is a general term. Three subtypes might be distinguished, namely mononeuropathy, mononeuropathy multiplex, or polyneuropathy. In peripheral neuropathies, it is essential to also determine whether the primary pathophysiology is of demyelinating or axonal type. Nerve injuries are traditionally classified according to the Seddon and Sunderland grading systems. The Seddon classification divides nerve injuries based on their severity into neurapraxia, axonotmesis, and neurotmesis. Neurapraxia, the mildest type of injury, involves only pathologic changes in the myelin sheath around the axon resulting in a conduction block and transient functional loss. It is associated with a good prognosis. In axonotmesis, the axon suffers injury resulting in wallerian degeneration of its distal segment; however, the supporting structures, including the perineurium and epineurium, remain intact. The prognosis for recovery remains good, but time is required for axonal regeneration (∼1 mm per day) from the point of injury to the target tissue. Neurotmesis is the most severe type of injury and refers to complete severance of the nerve. The functional loss is complete, and unless early surgical intervention is performed clinical recovery is not expected.
Sunderland proposed a 5-degree classification system with first-degree and second-degree injuries corresponding with neurapraxia and axonotmesis and third, fourth, and fifth degrees corresponding with endoneural, perineural, and epineural injuries, respectively.
Limitations of current diagnostic tests and imaging
In addition to the clinical examination, nerve conduction and electromyography (EMG) studies and quantitative neurosensory testing are most commonly used to assess peripheral neuropathies and nerve injuries. Although these techniques remain the reference standard, there are limitations. First, the information about the exact location, extent, and cause of nerve disorders is often limited. In addition, electrodiagnostic studies depend on operative and interpretative skills of the examiner and are not practical in patients with, for example, skin disorders or bleeding diathesis. In some studies, the positive predictive values are in the 30%–40% range and asymptomatic slowing of nerves is common. Nerve biopsy is often too invasive and may lead to considerable morbidity. Another fundamental issue is the evaluation of stage-specific interventions, to improve nerve regeneration. Key to this is the ability to follow the growth state of the neuron and associated axonal elongation/regeneration in the nerve (pathway) before its reconnection with target tissues. Current anatomic MRN techniques using fat-suppressed T2-weighted sequences are not able to sufficiently show nerve function and recovery. Therefore, further development of functional MRN technologies are of foremost interest if they can provide useful information not only on gross nerve morphology but also on microstructure, collagen integrity, demyelination, and, if possible, nerve function.
High-resolution MRN and new three-dimensional sequences
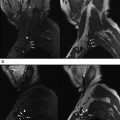
The increasing use of 3-T MR scanners, new phased-array surface coils, and parallel imaging techniques allow the acquisition of high-resolution and high-contrast images in short imaging times. Current state-of-the-art MRN provides detailed anatomic depiction of peripheral nerves and improved characterization of pathologic states ( Figs. 1 and 2 ).
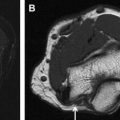
Axial T1-weighted and fluid-sensitive fat-suppressed T2-weighted images serve as the mainstay in MRN interpretation for prudent assessment of peripheral nerve imaging characteristics, such as signal intensity evaluation, course, caliber, fascicular pattern, size, and perineural fibrosis, or mass lesions. Normal nerves show intermediate signal intensity (similar to muscle) on T1-weighted images and intermediate to minimally increased signal intensity on T2-weighted images, depending on the amount of endoneural fluid and background fat suppression (see Fig. 1 ). MRN is a highly sensitive technique and may show abnormalities not revealed with electrophysiologic tests ( Fig. 3 ). However, the imaging abnormality may not recede immediately and completely despite patient improvement following treatment ( Fig. 4 ). Serial evaluation is nonetheless helpful to assess increase in size and signal abnormality in worsening cases. Focal alterations in nerve contour, course, and caliber are best depicted on longitudinal images reconstructed along the course of the nerve on dedicated imaging workstations using various techniques, such as multiplanar reconstruction, curved-planar reconstruction, and maximum intensity projection (MIP). For such reconstructions, imaging sequences should be acquired as near isotropic as possible (eg, 0.6–1 mm resolution). Only isotropic voxel sizes allow smooth reconstructions in any desired plane. Therefore, preferably three-dimensional (3D) sequences such as 3D fast (turbo) spin-echo (TSE) or 3D gradient-echo sequences should be used. The advantages of 3D TSE sequences (acronyms include VISTA [Phillips, Best, the Netherlands], SPACE [Siemens, Erlangen, Germany], CUBE [GE, Waukeesha, WI]) are that they combine high spatial resolution and, in theory, pure T2 contrast, if needed. The latter is important for the superior spatial depiction of the nerves coursing obliquely and for the interpretation of mild T2 because this could be an isolated sign of neuropathy. To enhance contrast/noise ratio between abnormal nerves with increased signal intensity and surrounding tissue, good fat-suppression techniques using spectral adiabatic inversion recovery (SPAIR) or short tau inversion recovery (STIR) techniques are frequently applied to those MRN sequences ( Fig. 5 ). Some vendors even allow the use of different strengths of fat saturation (eg, Phillips), which eases detection of abnormalities of thin nerves. In addition, 3D TSE sequences provide a familiar T2-weighted type of contrast. 3D TSE is useful for longitudinal depiction of nerve disorders, for the benefit of referring physicians and radiologists not reading MRN images on a routine basis.
Another new category of sequences to image peripheral nerves in the extremities are 3D diffusion-weighted (DW) reversed fast imaging with steady-state precession (3D DW-PSIF) sequences. This imaging technique has previously been used for the evaluation of cranial nerves and the lumbar plexus with good vascular suppression and has recently been applied to MR imaging of peripheral nerves. Besides retaining all the advantages of a traditional 3D sequence, 3D DW-PSIF provides a selective suppression of moving structures, including vascular flow, leading to an improved identification of nerves compared with standard two-dimensional T2-weighted images ( Fig. 6 ). It should therefore be incorporated in the MRN protocol whenever accurate nerve localization and/or presurgical evaluation are required.
In general, all new high-resolution and 3D sequences benefit from high field strength because of the significant increase in signal/noise ratio. If available, MRN studies should therefore be performed on MR units with 3.0 T or higher.
Whole-body MRN
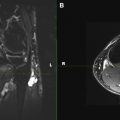
Multimodality scanners which are able to acquire coregistered structural and functional information, such as single-photon emission computed tomography (SPECT)/computed tomography (CT) and positron emission tomography (PET)/CT, play an increasingly important role in the evaluation of human disease. However, disadvantages of SPECT/CT and PET/CT are a long preparation time for the examination, exposure to ionizing radiation, and possible mismatch between anatomic and functional data sets caused by the patient repositioning. MR imaging is able to provide both anatomic and functional information within a single examination without these disadvantages. Whole-body MR imaging and whole-body DW imaging (DWI) can be performed in the same scanner, without patient repositioning. Furthermore, whole-body DWI does not require any contrast agent administration. In 2004, Takahara and colleagues showed the feasibility of whole-body DWI under free breathing. This concept is also known as DW whole-body imaging with background body signal suppression (DWIBS). Whole-body DWI, using the concept of DWIBS, may be a powerful adjunct to anatomic whole-body MR imaging, by detecting subtle lesions and pathologic changes in normal-sized structures, thanks to its high contrast/noise ratio (CNR). Feasibility studies showed the potential of DWI in visualizing the brachial plexus and the sacral plexus. Whole-body MR imaging also has a significant potential in assessing disease load and treatment response in cases of neurocutaneous syndromes ( Fig. 7 ). However, for any disorders, whole-body DWI should always be evaluated together with other (anatomic) whole-body MR imaging sequences, to avoid false-positive results. In addition, whole-body MRN is becoming feasible using DWI ( Fig. 8 ). These technical developments will likely play an important role in the assessment of disease burden in neurocutaneous syndromes, such as neurofibromatosis and schwannomatosis, and in diffuse polyneuropathy conditions such as Charcot-Marie-Tooth disease and chronic inflammatory demyelinating polyneuropathy (CIDP).