MAJOR SALIVARY GLANDS AND PERIPHERAL FACIAL NERVE
IMAGING APPROACH
Techniques and Relevant Aspects
Computed Tomography
Specific computed tomography (CT) protocols for various indications appear in Appendix A. The fundamental plane of the axial section should be the infraorbital meatal line for parotid studies. The gantry should always be angled to avoid dental-related artifacts. For submandibular or sublingual gland masses, sections should be parallel to the body of the mandible. It is better to position the patient’s head to allow for the optimal plane of section, but this can also be done with gantry angulation.
CT data sets should be obtained with about 1-mm collimation reconstructed at 1- to 3-mm slice thickness depending on the clinical situation. Such acquisitions will be suitable for adequate multiplanar reformations. If sections are too thin, there may be an important loss in low-contrast resolution that may cause a lesion to “hide” within the highly variable tissue density of the normal glands. Such occasionally poor contrast between salivary gland masses and the normal gland can be daunting. In any questionable case, magnetic resonance imaging (MRI) should be done to more confidently exclude a mass if one is strongly suspected clinically (Fig. 175.1). This issue actually makes MRI a better first choice for the evaluation of a parotid-region mass if there is no inflammatory component to the clinical presentation.
CT studies are normally performed with intravenous contrast. Precontrast images are generally not required with CT even when salivary gland stones might be present. If some confusion arises between possible focal enhancement and the presence of stones and that information is diagnostically important, the patient can be returned for delayed or a non–contrast-enhanced CT acquisition.
Magnetic Resonance Imaging
Specific magnetic resonance (MR) protocols for various indications appear in Appendix B. MRI is primarily done in the axial plane parallel to the infraorbital meatal line. The axial images are the mainstay for interpretation in parotid-region masses. Coronal and sagittal acquisitions are done selectively to evaluate the extent of a parotid lesion relative to the temporal bone and skull base and/or the facial nerve. Coronal images are extremely useful for evaluation of submandibular gland and sublingual gland pathology. Sagittal images are only occasionally useful or necessary.
Non–contrast-enhanced T1- and T2-weighted MR images may suffice for some indications. T1- and T2-weighted fat-suppressed techniques are useful and preferred unless there is a risk of significant field distortion artifacts, usually from dental sources. MRI should be done with 3-mm sections and a field of view of 12 to 16 cm. Diffusion-weighted images may be obtained since they may contribute information about the likely benign or malignant nature of the mass; however, it is unlikely that any serious clinical decision will be based on such data relative to that available from the clinical setting, anatomic imaging, and biopsy.
Contrast-enhanced MR may not be necessary in many cases; however, it is useful for evaluating clinically significant issues in malignant tumors such as perineural spread. Since it is not predictable when contrast might be useful, a major salivary gland study is generally done with acquisitions before and after contrast injection. Contrast-enhanced MR studies are clearly useful when cranial nerves V and VII must be evaluated, if the lesion is aggressive and/or if the cervical nodes are to be evaluated.
MRI sialography can be used to provide a relatively gross assessment of the parotid or submandibular ductal system (Fig. 175.2).
Ultrasound
Standard scanning techniques as described in Chapter 4 are used to examine the parotid and submandibular glands.
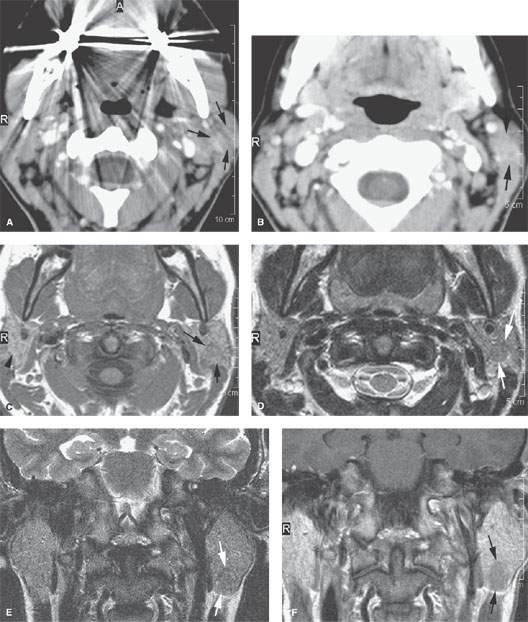
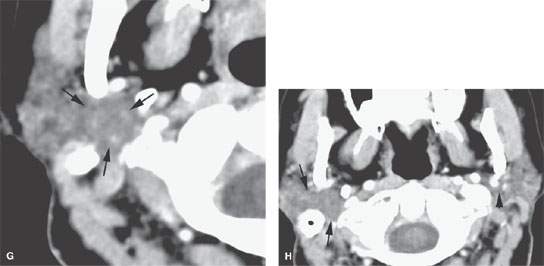
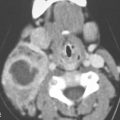
FIGURE 175.1. Imaging studies in two patients showing how mass lesions may be obscured within the major salivary gland both by artifacts and by inherent problems with tissue contrast on computed tomography (CT) and magnetic resonance (MR) studies. A–F: Contrast-enhanced CT in Patient 1 shows degradation due to dental-related artifacts (A), making the suspected mass in the parotid tail (arrows) impossible to identify accurately. In (B), the mass is still difficult to identify based on any density difference between it and surrounding parotid tissue. Clinically, there was a vague palpable fullness in the parotid gland; because CT was not definitive, MR was done. It could be reasonably argued that MR should have been done first. In (C), the non–contrast-enhanced T1-weighted (T1W) MR shows a very vague area of possible abnormality (arrows) in the parotid tail compared to the normal side (arrowhead). In (D), the T2-weighted (T2W) image shows no definite mass lesion but one possibly present in the left parotid tail (arrows). In (E), the T2W coronal image again shows a suspicious area in the parotid tail, although the mass is almost isointense to the remainder of the parotid tissue (arrow). In (F), the T1W coronal image with normal-enhancing glandular parenchyma reveals the less-enhancing mass in the parotid tail (arrows). This turned out to be an adenocarcinoma of the parotid gland. G, H: Patient 2, who had a fullness in the right parotid region noted clinically. Contrast-enhanced CT was done. In (G), it is very difficult to be sure if a mass different in density than the remaining parotid tissue is present in the deep portion of the gland based on this image. There appears to be fullness in the deep portion of the gland (arrows). In (H), the presence of a mass is more obvious when one compares the right side (arrows) to the left. On the right side, vascular structures can be seen to be displaced, and the area of the mass is manifest mainly by its effect on surrounding structures compared to the normal side (arrowhead). Note, however, that the mass is still essentially the same density as the remaining normal parotid tissue on both sides.
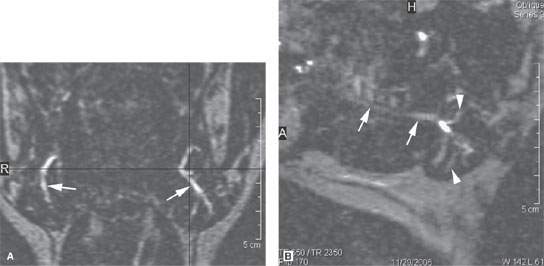
FIGURE 175.2. Sialography done with steady state magnetic resonance images. These images show a slightly dilated main submandibular duct (arrows in A and B) as well as a slightly dilated ductal system within the gland (arrowhead in B).
Radionuclide Studies
The method of use and constraints on the utility of radionuclide studies are discussed in Chapter 5.
Conventional Sialography
Conventional sialography is a simple and effective study, although its use has markedly diminished since the introduction of CT and further with MRI. It requires the use of sets of dilators and cannulas with a range of size that will adapt to the wide range of duct orifice size. The study is most efficiently done with fluoroscopic assistance and spot filming. It should not be done in glands that are acutely inflamed, even with the relatively nonirritating contrast agents currently available since they can worsen the condition to the point of creating an extravasation of potentially infected ductal contents and cause a parotid or extraparotid abscess that may not have been the eventual outcome of the disease.
Pros and Cons
General Approach
Magnetic Resonance and Computed Tomography
MR and CT are the most used imaging studies to evaluate a parotid-, submandibular/submental-, or sublingual-region mass. Such expensive studies are not usually necessary if the mass is discrete and freely mobile, as it can be followed clinically, biopsied, or removed safely is almost all instances depending on clinical circumstances (Fig. 175.3). If there is a hint of an inflammatory condition clinically in the presence of a mass, then CT is preferred over MR (Figs. 12.3A and 13.4 and Chapters 13 and 177). CT can detect small calculi and ductal dilatation as signs of an inflammatory pathology. MR sialography is not useful for masses and is only marginally useful in other conditions.
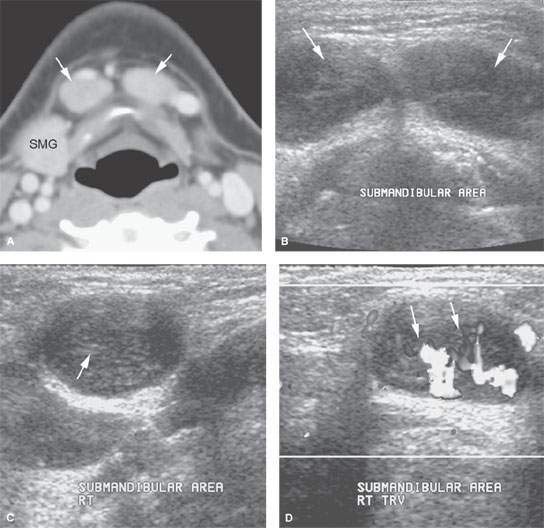
FIGURE 175.3. A 12-year-old patient presenting with a mass in the submental space. A: Computed tomography (CT) was done as the initial diagnostic examination. This study shows enlarged level 1A lymph nodes (arrows). (SMG, submandibular gland.) B–D: An ultrasound could have rendered the same result as the CT in (A) and spared the patient radiation or a more expensive magnetic resonance examination with contrast. In (B), axial images show what morphologically appears to be enlarged lymph nodes in level 1A (arrows). Images across the short axis of these nodes (C) show a likely area of more echogenicity in the node hilum. Morphologically, these most likely represent lymph nodes on the ultrasound study, confirmed in (D), which shows a predominately central and hilar flow pattern (arrows) in what was reactive submandibular adenopathy possibly due to catscratch disease.
Ultrasound
Ultrasound may be used to determine whether a superficial lesion is intrinsic or extrinsic to the parotid gland and to follow such a mass if it is likely a reactive node (Fig. 175.4) or if its benign nature is established by biopsy and definitive surgery is not elected. Ultrasound may be used to guide percutaneous biopsies of major salivary gland masses that cannot be done by palpation alone and may help to improve the diagnostic yield of those procedures by ensuring that the sampling is from the mass and not surrounding normal gland. The combination of ultrasound and biopsy can be a very quick and cost-effective way to evaluate a superficial mass that is likely to be benign or related to an enlarged periparotid or intraparotid lymph node.
Ultrasound may be used effectively for the evaluation of parotid inflammatory disease to exclude obstructive sialoadenitis and to identify points of main duct obstruction in dilated ductal systems (Fig. 175.5).
Radionuclide Studies
Radionuclide studies are not used routinely for the evaluation of salivary gland masses. The constraints on its utility are discussed in more detail in Chapter 5 and with regard to glandular neoplasms in Chapter 22. Technetium, iodine, and fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) activity is frequently seen in normal glands (Figs. 5.9 and 5.20B). FDG uptake is variable in both benign and malignant salivary gland neoplasms, somewhat limiting the utility of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) studies in patients with salivary gland masses (Fig. 5.12).
Conventional Contrast Sialography
Traditional contrast sialography remains useful to assess ductal anatomy for strictures and the few intraductal stones that are not visible at CT. Stones will be missed on MRI most of the time (Fig. 175.6).
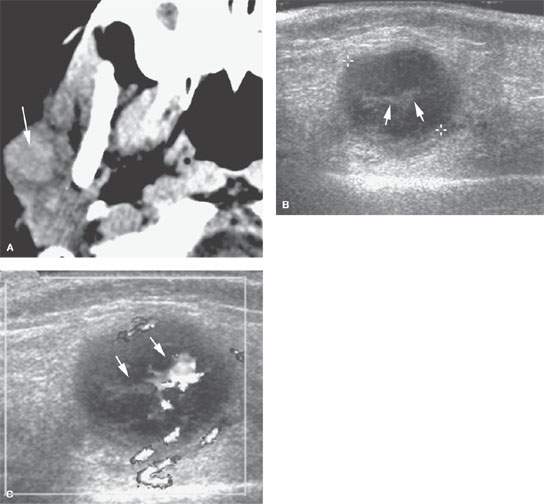
FIGURE 175.4. A 6-year-old patient with fullness in the right parotid gland. A: Computed tomography was done as the initial examination and showed a mass in the right parotid gland. The mass was easily palpable and freely moveable. Since this was felt to almost certainly be an abnormal lymph node in a patient of this age, an ultrasound was done to confirm that impression and to help with follow-up. B: Typical morphology of an enlarged intraparotid lymph node with central echogenic hilar structures (arrows). C: Color flow Doppler image showing hilar hypervascularity, which is fairly typical of a reactive lymph node. Based on this imaging, it was elected to follow the patient. The lymph node could have been biopsied. The node resolved without further treatment. (NOTE: In superficial parotid masses and ones that are freely moveable, ultrasound can be used to determine whether an enlarged node is the likely etiology and can help to guide biopsy perhaps more accurately than by freehand techniques.)
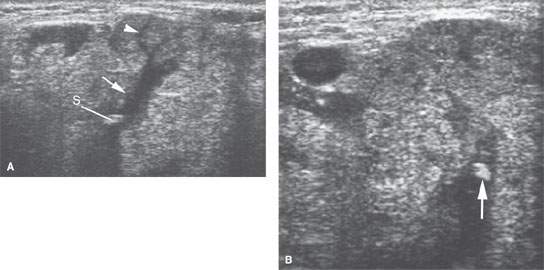
FIGURE 175.5. A patient with low-grade tenderness and swelling in the submandibular region. The two ultrasound images in (A) and (B) show high-level echoes within a dilated ductal system (arrows). These findings confirmed the clinical suspicion of a stone causing obstructive sialoadenitis (arrowhead).
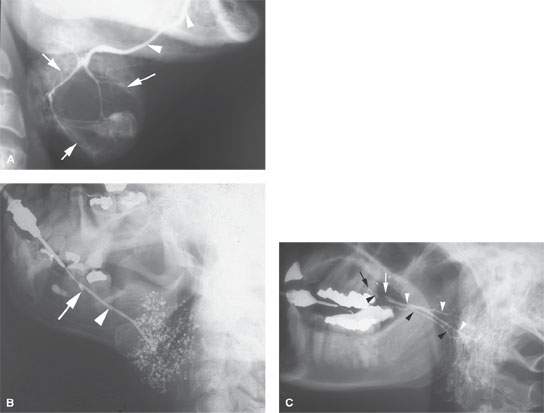
FIGURE 175.6. Conventional sialography in three different patients. A: Patient 1. A submandibular sialogram showing the course of the submandibular duct in the floor of the mouth (arrows). This can be judged as a technically adequate study by the excellent filling of the duct and the parenchymal stain obtained. In this patient, the parenchymal stain is replaced by a central mass displacing the ducts and causing a lack of glandular staining centrally. These findings were consistent with an intrinsic mass in the submandibular gland. This study was done about 30 years ago. Sialography is not currently used to evaluate salivary gland masses. B: Patient 2. A submandibular sialogram showing the cannula placed in the opening in the submandibular duct in the anterior floor of the mouth (arrow). Note the excellent anatomic detail of the main submandibular duct (arrowhead). The parenchymal pattern of punctuate sialoadenitis is very well demonstrated. (NOTE: Such findings can be demonstrated on magnetic resonance (MR) sialography but not with equivalent detail. Also, this conventional sialogram was easy to perform and much less expensive than a MR “sialogram.” If the ductal system needs to be studied definitively and the patient does not have acute or subacute inflammatory disease, then conventional sialography remains an excellent choice of diagnostic studies. C: Patient 3. Lateral view of a conventional sialogram of the parotid gland. The appearance of the study is unusual because both parotid ductal systems and glands are being shown at once. On one side, there is a stricture (white arrow) in the distal main parotid duct, resulting in dilatation of that entire ductal system (white arrowheads). This resulted in delayed emptying of that parotid gland following removal of the cannula. Subsequently, the opposite parotid gland duct was cannulated (black arrow) and the normal ductal system shown. (NOTE: The point of this case is to demonstrate that conventional sialography produces a superb rendering of ductal systems of the salivary glands when the ductal systems need to be studied definitively. The demonstration of strictures and stones with this degree of clarity is not within the capability of MR sialography.)
Specific Indications
Parotid-Region Masses
Determine the origin and extent of a parotid-region mass: If the margins of a mass are not clear after physical examination, then CT or MR should be done. This will first help to determine whether the mass point of origin is intrinsic or extrinsic to the gland. This factor alone markedly alters the course of management in many patients (Figs. 6.9, 6.15, 7.12, and 175.7) since “parotid masses” are frequently of nonparotid origin (Chapter 179). Imaging can quickly narrow the diagnostic possibilities by excluding many of the extrinsic causes of parotid-region masses.
Define the relationship of an intrinsic parotid mass to the facial nerve and its major branches: This can, depending on interpretive expertise, be simply limited to whether the mass is superficial or deep to the main trunk of the facial nerve (Chapter 179). With little effort, it is possible to refine this by describing the position of the mass in the gland relative to the usual surgical landmarks used to identify and dissect the facial nerve and its main branches1–3 (Chapter 179).
Characterize the type of intrinsic lesion present and the degree of invasiveness both within and beyond the parotid gland: To evaluate the full extent of the lesion, determine whether it has morphology or associated findings suggestive of an invasive and/or malignant process. This includes the margins of these lesion, internal signal characteristics on T2-weighted images, and perhaps behavior on diffusion-weighted imaging (DWI). Metastatic adenopathy and perineural spread are almost sure signs of a malignant tumor.
Determine the extent of perineural tumor spread in patients with known or suspected malignancies: Evaluate for gross perineural spread of tumor along the facial, auriculotemporal, and mandibular (V3) nerves distally and to their most proximal connections to the brain stem. Negative imaging studies do not exclude perineural spread.
Submandibular- and Sublingual-Region Masses
CT and MR are also essential for evaluating masses in the region of the submandibular and sublingual glands. They primarily accomplish the same goals as stated for parotid-region mass lesion studies except for those related to the facial nerve. As in the parotid region, if there is an inflammatory component to the clinical presentation, CT is the preferred starting point.
MR or CT is almost always capable of differentiating an intrinsic submandibular gland neoplasm from an extrinsic mass (Chapter 182). Occasionally, other masses, such as a ranula, dermoid cyst, or thyroglossal duct cyst or rare lesions of neural or mesenchymal origin, will mimic a submandibular or sublingual gland or nodal lesion. Inflammatory disease will usually manifest as abnormal glandular enhancement, ductal dilatation, and/or the presence of stones (Chapters 178, 181, and 183).
The mass may be malignant if it is intrinsic to the gland, and the study should be extended to include the cervical lymph nodes.
Ultrasound may be substituted in selected cases for the submandibular gland, much the same as it is used in the parotid gland (Fig. 175.3). The value of ultrasound in the sublingual gland and space may be marginal compare to its abilities in the other major salivary glands.
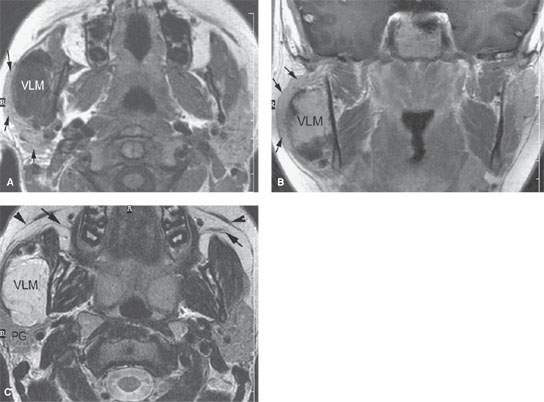
FIGURE 175.7. The evaluation of parotid and submandibular masses is greatly aided by imaging with computed tomography, magnetic resonance (MR), and ultrasound (if desired) depending on the clinical situation. This contrast-enhanced MR study was done in a 19-year-old patient with a right parotid region mass. A: T1-weighted (T1W) axial images without contrast showing the parotid gland (arrows) to be stretched over the venolymphatic malformation (VLM) in the right masseter muscle. Obviously, this markedly changes management. B: Contrast-enhanced T1W image in the coronal plane showing the parotid gland (arrows) again stretched over the now-enhancing venolymphatic malformation (VLM). C: T2-weighted axial image clearly localizing this lesion to the masseter muscle and separate from the parotid gland (PG). This image also illustrates the position of the superficial musculoaponeurotic system layer of the face, which should not be mistaken for the main parotid ducts (arrows).
Inflammatory Disease
CT is preferred in cases of acute major salivary gland pathology. Such imaging can demonstrate significant ductal obstruction and almost all obstructing stones when that is the etiology of the inflammatory disease (Figs. 12.3A and 13.4 and Chapters 13 and 177). Contrast-enhanced CT can also detect early parotid abscess as opposed to diffuse nonspecific glandular enhancement. CT will also identify infectious or inflammatory processes arising from surrounding structures, most commonly dental infections. MRI lacks the bone detail to confirm such unexpected etiologies when they are the source of infection rather than a salivary gland. While MRI with MR sialography may help in acute inflammation, it is generally deficient compared to CT.
In chronic inflammatory disease, imaging is usually of limited clinical benefit. CT can certainly confirm the presence of stones and ductal dilatation and gland atrophy. If imaging shows the disease to be bilateral or otherwise involve multiple glands, it can suggest a systemic autoimmune process or sarcoidosis as a potential diagnosis; the presence of such a disease is typically known before such imaging.
MR sialography has been suggested as an alternative to conventional sialography to confirm the presence of Sjögren disease; this seems to be an unnecessary expenditure of resources for a disease that is diagnosed clinically, by laboratory studies, and, when necessary, a very much less expensive and more specific lip biopsy. MR sialography can be substituted for conventional sialography to evaluate the gross morphology of the ductal system as an aid to surgical decision making in patients who have recurrent disease and a stricture needs to be identified. Conventional sialography can be used for this purpose and is less expensive. Conventional sialography should not be done when there is evidence of acute or subacute glandular infection.
Ultrasound can also be used to screen for obstruction and to some extent intraglandular complications of inflammatory disease; in some cases, it may prove definitive enough for disposition and save a CT or MR examination (Fig. 175.5).
Controversies
The use of MR sialography has been proposed for the diagnosis and evaluation management of patients with Sjögren disease or syndrome. This may make sense in practices where cost is not an issue. Yet it does not seem to make sense given the typical clinical approach to diagnosis and management of this disease. Moreover, MR sialography images (Fig. 175.2) are considerably inferior to conventional sialography (Fig. 175.6) and cannot be used to exclude small filling defects, stones, or strictures.
DWI data may differentiate benign and malignant salivary gland tumors with reasonable accuracy. This ability would seem to be limited by the size of the mass to some extent. Moreover, it is unlikely that any critical decision making would result from DWI data. It costs nothing and can add information, so there is no harm in doing the analysis. There is only potential harm in relying on the data from DWI as a critical point in the management pathway.
NORMAL ANATOMY
The parotid is the largest of the major salivary glands, with rather complex and important anatomic relationships (Figs. 175.8–175.11). There is some controversy with regard to whether the parotid has a distinct fascial capsule. The deep cervical fascia penetrates the gland in multiple areas and produces inconstant “lobes.” The lobes, being truly inconstant anatomically, require that various regions of the parotid glands be spoken of in surgical jargon as “areas” or “parts” or “lobes” of the gland; the usage of these modifiers varies, but they all essentially mean the same thing depending on the term preferred by the clinician involved. The commonly used differentiation of superficial and deep lobes, while clinically very useful, is still arbitrary, being based on the position of the main trunk of the facial nerve.
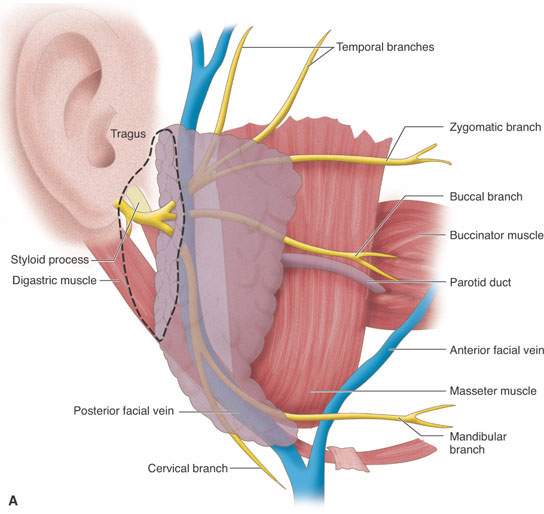
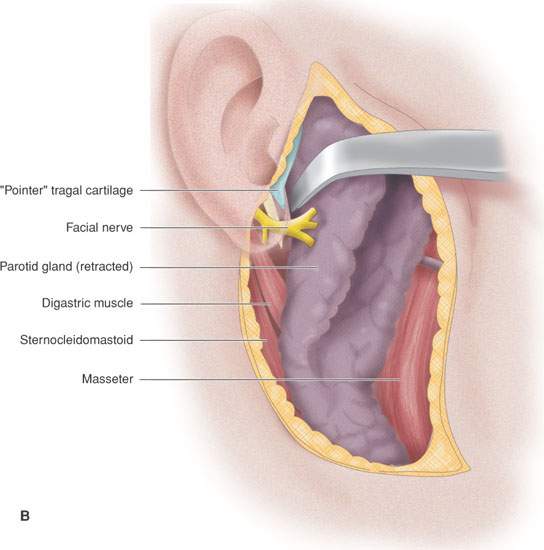
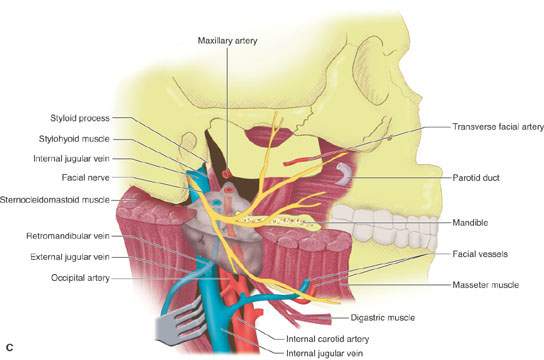
FIGURE 175.8. A series of images showing important anatomic relationships of the parotid gland, especially with regard to the facial nerve. A: Note the relationship between the main trunk of the facial nerve as it enters the back surface of the parotid gland to the surrounding anatomic structures, including the digastric muscle and styloid process. Also note the position of its more distal branches relative to structures of the face. B: This diagram emphasizes surgical landmarks used to identify the main trunk of the facial nerve and carry out a dissection of that nerve, protecting it from harm during parotid surgery. These landmarks can be easily identified on imaging studies. In particular, note the pointer of the tragal cartilage, also known as the tragal pointer, and the digastric muscle. C: A diagram illustrating the deeper relationships of the facial nerve as it extends through the parotid gland relative to the vessels that travel through the gland and then on peripherally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree