Etiology
Malignant liver tumors can be classified either by cell of origin as hepatocellular, cholangiocellular, or mesenchymal or by site of origin as primary or secondary. This chapter will describe the most frequently encountered malignant hepatic tumors arising in the noncirrhotic liver, including hepatocellular carcinoma (HCC), fibrolamellar HCC, epithelioid hemangioendothelioma (EHE), angiosarcoma, and metastatic disease. Also discussed are other rare primary liver tumors, such as lymphoma and hepatoblastoma. HCC arising in cirrhosis and intrahepatic cholangiocarcinoma are discussed elsewhere in this text.
Prevalence and Epidemiology
Metastases are the most common malignant neoplasm of the liver, with an incidence of 40% in cancer patients at the time of death. Although HCC—one of the most common causes of cancer death worldwide—usually occurs in the setting of cirrhosis, in approximately 10% of cases this tumor occurs without cirrhosis or other known risk factors. Other primary malignant mesenchymal (i.e., angiosarcoma and EHE), hepatocellular (i.e., hepatoblastoma), or lymphoid (i.e., primary or secondary lymphoma) tumors are much rarer, accounting for only 1% to 2% of all primary malignant liver neoplasms.
Prevalence and Epidemiology
Metastases are the most common malignant neoplasm of the liver, with an incidence of 40% in cancer patients at the time of death. Although HCC—one of the most common causes of cancer death worldwide—usually occurs in the setting of cirrhosis, in approximately 10% of cases this tumor occurs without cirrhosis or other known risk factors. Other primary malignant mesenchymal (i.e., angiosarcoma and EHE), hepatocellular (i.e., hepatoblastoma), or lymphoid (i.e., primary or secondary lymphoma) tumors are much rarer, accounting for only 1% to 2% of all primary malignant liver neoplasms.
Pathophysiology
Malignant liver lesions may occur anywhere in the liver. The ability of computed tomography (CT) and magnetic resonance imaging (MRI) to show hepatic tumors is enhanced by the dual blood supply of the liver. Although liver tumors generally receive nearly all their blood supply from the hepatic artery, after bolus intravenous injection of a contrast agent some of them enhance more than the surrounding liver parenchyma (hypervascular tumors) in the hepatic arterial phase, whereas others will be best depicted as low-attenuation or low-intensity lesions (hypovascular) against the background of enhanced liver during the portal venous phase.
Liver Function
Tumor markers such as alpha-fetoprotein, protein induced from the absence of vitamin K (PIVKA), carcinoembryonic antigen (CEA), and cancer antigen 19-9 (CA 19-9) are commonly used for differentiating focal liver lesions, although their role for diagnosis is controversial. Laboratory examination is nonspecific and can reveal increased levels of alkaline phosphatase and transaminases or minor elevation of bilirubin levels.
Imaging
Computed Tomography
The multiphasic imaging capability of multidetector CT (MDCT) allows different protocols to be set for both hypovascular and hypervascular lesions. The advantages of MDCT include high speed with less motion artifact (i.e., improved temporal resolution), submillimeter slice thickness with true isotropic imaging, ease of image interpretation, and the ability to cover large volumes and create multiplanar reformatted images. Sagittal, coronal, or curved multiplanar reformatted images can better delineate those small subcapsular lesions in the dome of the liver that might not be well depicted with transverse imaging alone. In patients who are candidates for hepatic resection, CT angiography yields an excellent depiction of the relationship of the lesion to intrahepatic vessels.
Magnetic Resonance Imaging
The major advantages of MRI over CT are higher contrast resolution; better ability to detect, characterize, and quantify both intrahepatic and intralesional fat and iron; and the use of both extracellular and hepatobiliary contrast agents. The most diagnostically important information is usually obtained from multiphase gadolinium-enhanced, fat-suppressed three-dimensional T1-weighted gradient recalled echo MRI, including a properly timed hepatic arterial phase, followed by portal venous and delayed phases. T2-weighted imaging is not used for lesion detection but rather for lesion characterization. Precontrast T1-weighted in-phase and opposed-phase imaging allows assessment of lipid and iron content within the liver or lesion. With the widespread of hepatobiliary contrast media (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid [Gd-EOB-DTPA] and gadobenate dimeglumine [Gd-BOPTA]) and diffusion weighted imaging, MRI currently represents the modality of choice for the detection of hepatic metastases, with reported diagnostic accuracy values exceeding those of either ultrasonography or MDCT.
Ultrasonography
Although ultrasonography is frequently the first-line modality for imaging of the liver because of low costs and widespread availability, its role is currently limited in the evaluation of patients with malignant liver tumors and must be invariably supplemented with either MDCT or MRI. By providing insights on lesion vascularity, ultrasound contrast agents have recently shown the potential to improve both detection and characterization of a lesion.
Positron Emission Tomography with Computed Tomography
Because most malignant tumors show abnormally high fluorodeoxyglucose (FDG) metabolism, positron emission tomography (PET) provides a differentiating clue between malignant and benign hepatic lesions. Potential sources of confusion may occur with a minority of liver abscesses, which may simulate malignant lesions secondary to increased FDG uptake (false-positive finding). Conversely, false-negative results may occur with smaller or well-differentiated HCCs, which may demonstrate only subtle increase in FDG uptake. Besides its well-established role in lesion detection, PET also can provide important clues for tumor staging and early detection of tumor recurrence after therapy.
The role of imaging in the approach to malignant focal liver lesions is to determine the following ( Table 37-1 ) :
- •
Number, size, and location
- •
Differentiation of benign versus malignant
- •
Differentiation of primary versus secondary
- •
Whether the background liver shows normal or altered morphology (e.g., chronic liver disease)
- •
Whether the background liver shows fatty infiltration
- •
Indications for biopsy (especially in smaller lesions)
- •
Suitability of resection (relationship of the tumor to surrounding vessels)
- •
Tumor staging (rule out extrahepatic metastatic disease)
- •
Reliable follow-up after treatment
| Tumor | Sex | Age | Capsule | Size | Number | Calcification | Fat | Scar | Bleed | Necrosis | Associated Signs and Predisposing Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelioid hemangioendothelioma | F > M | 4th-5th decade | No | Variable | Multifocal | 13% | No | No | No | No | Capsular retraction Subcapsular location |
| Angiosarcoma | M > F | 6th-7th decade | No | Variable | Multiple nodules or large mass | Rare | No | No | Yes | Yes | Thorotrast |
| Fibrolamellar carcinoma | M = F | 2d-3rd decade | 35% | Large | Usually solitary | Central (68%) | No | Yes (80%) Hypo on T2-weighted | No | Yes | Lymphadenopathy |
| HCC in noncirrhotic liver | M > F | 5th-6th decade | 51% | Large | One or few | Peripheral (28%) | 10% | No | Rare | Yes | Hepatitis B and C |
| Hepatoblastoma | M > F | Infants and children <3 yr | No | Large | Single or multinodular | 30% | No | No | Yes | Yes | Genetic |
| Lymphoma | M > F | 6th-7th decade | No | Variable | Usually solitary | Rare (10%) | No | No | No | No | Multiorgan involvement and lymphadenopathy |
| Hypervascular metastases | M = F | Variable | No | Variable | Multiple | Rare (neuroendocrine) | No | Rare (neuroendocrine) | No | Yes | Primary malignancy |
| Hypovascular metastases | M = F | Variable | No | Variable | Multiple | Rare (colon) | No | No | No | Yes | Primary malignancy |
Specific Lesions
Epithelioid Hemangioendothelioma
Etiology
There are no known risk factors for hepatic EHE.
Prevalence and Epidemiology
Hepatic EHE is a low to intermediate grade malignant vascular neoplasm with an intermediate clinical course between that of cavernous hemangioma and malignant angiosarcoma. EHE shows a slight female predominance (male-to-female ratio, 2 : 3), with a peak incidence of approximately 50 years of age.
Clinical Presentation
Clinical manifestations are nonspecific and vary from asymptomatic patients to patients with portal hypertension or hepatic failure. The most common findings are weakness, anorexia, weight loss, right upper quadrant pain, and hepatomegaly. Unusual manifestations includes acute abdomen from rupture of the tumor hemoperitoneum, Budd-Chiari–like syndrome, portal hypertension, and liver failure as a result of extensive replacement of liver parenchyma by the tumor.
Pathophysiology
Characteristically, EHE demonstrates a peripheral, subcapsular location, although in advanced cases lesions may grow to almost replace the entire liver. Compensatory hypertrophy of unaffected hepatic parenchyma can be seen in patients with extensive liver involvement.
Pathology
Typically, EHE manifests as multiple lesions, ranging from 1 to 3 cm in diameter. On sectioning, lesions are white to tan and firm, with a fibrous, relatively hypocellular center surrounded by a rim of hyperemic, actively proliferating viable tissue. An additional peripheral hypovascular halo may be observed at the interface between the tumor and the surrounding hepatic parenchyma and has been related to a narrow avascular zone secondary to tumor occlusion of hepatic sinusoids and small vessels. Larger lesions are generally confluent, thus forming aggregate masses that may sometimes contain coarse calcifications. Demonstration of the vascular or endothelial origin of the tumor is critical for diagnosis and requires immunostaining for endothelial markers, including factor VIII–related antigen, CD31, and CD34.
Liver Function
Increased alkaline phosphatase activity may be present in approximately two thirds of patients. Tumor marker levels, such as alpha-fetoprotein and CA 19-9 levels, are generally unremarkable.
Imaging
Computed Tomography.
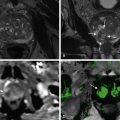
CT findings reflect the gross appearance of the tumor and typically demonstrate multiple, peripheral, and partially confluent liver masses. Capsular retraction can be seen when lesions abut the liver surface. On precontrast CT, EHE is generally hypoattenuating compared with the liver, with the only exception being occasional intralesional calcifications. On contrast-enhanced CT, EHE shows a very characteristic target-type enhancement pattern with a central hypoattenuating area, which corresponds to the central fibrous core, surrounded by a peripheral thick enhancing ring and an outer hypoattenuating halo, which corresponds to the peripheral viable tumor and the avascular zone of transition, respectively ( Figure 37-1 ).
Magnetic Resonance Imaging.
On T1-weighted MRI, EHE shows hypointensity with a markedly hypointense central area. On T2-weighted MR images, EHE shows a characteristic target appearance with a markedly hyperintense central area and a mild hyperintense peripheral rim (see Figure 37-1 ). On diffusion weighted images, the central area shows restricted diffusion, while the peripheral rim shows intensity decrease at high b-value. On dynamic images, the tumor enhancement pattern is substantially comparable to that on CT.
Ultrasonography.
EHE appears as lobulated confluent hepatic lesions with variable echotexture on gray-scale ultrasonography and with most lesions being hypoechogenic.
Positron Emission Tomography With Computed Tomography.
18 FDG-PET/CT has shown some utility for detection of EHE recurrence after resection.
Imaging Algorithm.
An imaging algorithm is provided in Figure 37-14 at the end of this discussion.
- •
Multiple round lesions or large confluent masses
- •
Target appearance (“bull’s eye”)
- •
Subcapsular location with retraction of the overlying capsule
Differential Diagnosis
Absence of a known history of a primary tumor may be helpful in ruling-out metastatic disease. Because EHE may occasionally show delayed enhancement, lack of a history of cirrhosis helps in differentiating it from peripheral cholangiocarcinoma. Positive imaging findings, in addition to features such as occurrence in younger adults, relative indolent progression of the disease, and the presence of numerous intrahepatic tumors with a good clinical condition are suggestive of EHE.
EHE can be confidently differentiated from hemangioma based on tumor enhancement as well as demonstration of retraction of the adjacent liver capsule ( Table 37-2 ). In cases in which retraction of the liver capsule overlying a lesion is noted, absence of morphologic changes of cirrhosis and regenerative nodules is helpful to differentiate these lesions from confluent hepatic fibrosis and absence of biliary dilatation is key to differentiate them from peripheral cholangiocarcinoma ( Table 37-3 and Figure 37-2 ). The definitive diagnosis requires core biopsy and histologic analysis, because the typical target sign observed in EHE can be mimicked by metastatic disease ( Figure 37-3 ).
| Features | Hemangioma | EHE | Angiosarcoma |
|---|---|---|---|
| Sex | F > M | F ≥ M | M > F |
| Age | 2nd-5th decade | 4th-5th decade | 6th-7th decade |
| Location | Variable | Subcapsular | Diffuse liver involvement |
| Shape | Round/oval | Round | Ill-defined |
| Number | Single > multiple | Multiple | Multiple |
| Capsular retraction | No | Yes | No |
| Central scar | Rare (larger lesions) | No | No |
| Calcifications | Only large lesions | Uncommon | Uncommon |
| Enhancement | Centripetal | Low degree | Bizarre, centrifugal |
| Necrosis | No | No | Yes |
| Features | Peripheral Cholangiocarcinoma | Focal Confluent Fibrosis | EHE | Metastases | Hemangioma |
|---|---|---|---|---|---|
| Sex | F = M | M > F | F ≥ M | F = M | F > M |
| Age | 6th-8th decade | 6th-7th decade | 4th-5th decade | Any age | 2nd-5th decade |
| Predisposing factors | Primary sclerosing cholangitis | End-stage cirrhosis | None | Primary malignancy | None |
| Number | Single | Single | Multiple | Multiple | Single > multiple |
| Delayed enhancement | ++ | ++ | ± | ± | +++ |
| Central calcifications | No | No | Uncommon | Uncommon (mucinous primary) | Uncommon (larger lesions) |
| Capsular retraction | ++ | ++ (Advanced cirrhosis) | ++ | After chemotherapy | No |
| Necrosis | ++ | − | ± | ++ | − |
| Enhancement on portal venous phase | + (Ring) | Rare (trapped vessels) | − | ± | ++ (Nodular, peripheral, discontinuous) |
Treatment
Medical Treatment.
At present, the roles of radiation and chemotherapy are still undetermined.
Surgical Treatment.
Surgical resection and liver transplantation are considered the treatments of choice. Liver transplantation is beneficial in patients with multiple lesions and extensive involvement of liver parenchyma.
- •
EHE is a low-grade malignant primary tumor.
- •
CT and MRI are the best diagnostic tools but cannot provide a definitive diagnosis.
- •
Biopsy is required for definitive diagnosis.
- •
Liver resection and transplantation are the treatments of choice.
Angiosarcoma
Etiology
Although several conditions, such as hemochromatosis, von Recklinghausen’s disease, and environmental carcinogens (i.e., vinyl chloride, thorium dioxide, and arsenic), may favor the development of angiosarcoma, currently most cases occur in patients without known associated risk factors.
Prevalence and Epidemiology
Angiosarcoma is a high-grade malignant neoplasm of endothelial cells. This tumor represents the most common sarcoma of the liver. The peak age incidence is in the sixth and seventh decades, with a male-to-female ratio of 3 : 1.4
Clinical Presentation
Angiosarcomas typically manifest at an advanced stage with hepatomegaly, ascites, abdominal pain, and weight loss. Sudden onset with acute symptoms may result from spontaneous tumor rupture and subsequent hemoperitoneum.
Pathophysiology
Angiosarcoma manifests as a single or a multifocal lesion, and involves both liver lobes.
Pathology
Typically, angiosarcoma is a well-circumscribed lesion with a variegated appearance resulting from areas of hemorrhage and necrosis. On histologic examination, tumor cells demonstrate a preferential growth along sinusoids, terminal hepatic venules, and portal vein branches that lead to progressive disruption of liver cell plates with the development of blood-filled cavitary spaces of varied size. Invasion of terminal hepatic venules and portal vein branches also leads to atrophy, infarction, and necrosis of hepatic parenchyma.
Liver Function
The most reliable abnormalities include increased serum alkaline phosphatase activity, hyperbilirubinemia, and prolonged prothrombin time.
Imaging
Angiosarcomas show an aggressive behavior with metastases to spleen, lung, bone marrow, portohepatic nodes, and peritoneum. A rare complication is tumor rupture, which results in acute hemoperitoneum. Because of the tumor vascularity and associated coagulopathy and thrombocytopenia, liver biopsy is associated with significantly high morbidity and mortality rates.
Computed Tomography.
On precontrast CT, angiosarcoma is hypoattenuating or isoattenuating to surrounding liver. Focal hyperattenuating areas can be seen secondary to intralesional hemorrhage. On contrast-enhanced CT, lesions have a variable appearance. In some cases, angiosarcoma lacks obvious enhancement, whereas some other times it shows irregular arterially enhancing foci, which increase in size on portal venous and delayed phases ( Figure 37-4 )
Magnetic Resonance Imaging.
Because angiosarcomas are predominantly composed of blood-filled tumor cavities, they show heterogeneous and markedly high signal intensity on T2-weighted images and very low signal intensity on T1-weighted images. Intralesional hemorrhagic foci can be seen as focal areas of bright signal intensity on T1-weighted images. On low- and high b-value diffusion weighted images, angiosarcoma shows heterogeneous high intensity, with high intensity on apparent diffusion coefficient (ADC) map. On gadolinium-enhanced MRI, the tumor enhancement pattern is substantially comparable to that at CT.
Ultrasonography.
Angiosarcomas manifest as heterogeneously hyperechoic lesions.
Positron Emission Tomography With Computed Tomography.
Lesions show markedly increased accumulation of 18 FDG compared with the surrounding liver parenchyma. This technique is particularly sensitive for the detection of distant metastases.
Imaging Algorithm.
An imaging algorithm is provided in Figure 37-14 .
- •
Multifocal nodules
- •
Heterogeneous enhancement
- •
Progressive enhancement over time
- •
Distant metastases
Differential Diagnosis
Association with hemochromatosis or von Recklinghausen’s disease or exposure to Thorotrast and arsenic can raise the suspicion of angiosarcoma. BizaFrre shape of the enhancing foci, large size of dominant lesions, multifocal distribution, and intratumoral hemorrhage are useful differentiating features from those of cavernous hemangioma (see Table 37-2 ).
Treatment
Medical Treatment.
Chemotherapy or antiangiogenic therapy may be performed in patients with diffuse liver involvement that is not amenable to surgical treatment.
Surgical Treatment.
Combined surgery and radiation therapy can offer the best chance of treatment, although the tumor is invariably associated with a poor outcome (5-year survival rate, 37%).
- •
Angiosarcoma is the most common malignant mesenchymal tumor of the liver.
- •
It may be difficult to differentiate from other primary or secondary neoplasms.
- •
Multiphasic contrast-enhanced CT and MRI are the best diagnostic tools.
- •
Outcome is poor.
- •
The tumor can recur after surgery.
Hepatocellular Carcinoma in the Noncirrhotic Liver
Etiology
Although predisposing factors such as hepatitis, viral infection, or alcohol abuse have been reported in some cases, no underlying liver disease can be found in the majority of patients. Nonalcoholic fatty liver disease may represent the underlying causative factor in some instances.
Other causes include exposure to genotoxic substances (e.g., aflatoxin B1) and hereditary disorders (e.g., hemochromatosis and Wilson’s disease).
Prevalence and Epidemiology
In a substantial number of cases, noncirrhotic HCC may arise de novo in an otherwise normal liver. Unlike cirrhotic HCC, noncirrhotic HCC shows a bimodal age distribution, peaking at the 2nd and 7th decades. Patients have a better prognosis as well as a longer survival rate than patients with HCC in the cirrhotic liver.
Clinical Presentation
Because the disease course is generally indolent and tumor surveillance is not performed, tumor size is typically large at diagnosis. At presentation, the most common signs and symptoms include abdominal pain, distention, weight loss, and anorexia. In a limited number of patients without referred symptoms, the tumor may be incidentally discovered at imaging studies for unrelated reasons.
Pathophysiology
Noncirrhotic HCC occurs predominantly in the right hepatic lobe.
Pathology
Noncirrhotic HCC typically manifests as a large, predominantly solitary or dominant mass with satellite lesions. Lesions may be well-defined and partially encapsulated, with areas of hemorrhage, macroscopic fat, and necrosis. Invasion of the portal vein or the biliary ducts and metastases to abdominal lymph nodes are occasionally observed. Despite the large size of lesions, most noncirrhotic HCCs are well to moderately differentiated at histologic analysis. This finding correlates well with the favorable prognosis of this tumor compared with that of noncirrhotic HCC.
Liver Function
Although serologic levels of alpha-fetoprotein are abnormally increased in 65% of cases, in a consistent number of patients this tumor marker is within normal values (20 mcg/L or less).
Imaging
Noncirrhotic HCC comes to clinical attention because of a palpable abdominal mass, abdominal pain, distention, weight loss, anorexia, or cachexia.
Computed Tomography.
On precontrast CT, noncirrhotic HCC manifests as a large, dominant lesion that is hypoattenuating compared with the surrounding liver, with the exception of occasional peripheral calcification. With contrast-enhanced CT, noncirrhotic HCC shows heterogeneous, moderate enhancement during the hepatic arterial phase, followed by washout (i.e., the lesion is hypoattenuating relative to the liver) during the portal venous and delayed phases. Areas of necrosis or hemorrhage are frequently depicted as nonenhancing intralesional foci ( Figure 37-5 ). Tumor invasion of the portal vein, hepatic veins, and biliary ducts is common.