Small bowel neoplasms remain a diagnostic challenge for radiologists and clinicians. The small bowel represents 75% of the total length of the gastrointestinal tract and more than 90% of the mucosal surface, but less than 2% of all gastrointestinal malignancies originate in the small bowel. Malignant tumors of the small bowel may arise from the mucosal epithelium, lymphoid tissue, blood vessels, nerves, and muscle. Secondary involvement of the small bowel is more common than primary malignancies. Considerable delay in the diagnosis of small bowel malignancies leads to low survival rates. Increased awareness of the small intestine as a potential source of nonspecific abdominal complaints or chronic anemia and the selection of the most accurate diagnostic tests would lead to improvement of a patient’s prognosis.
In this chapter both secondary and primary malignancies of the small bowel are reviewed, with an emphasis on their imaging findings ( Table 28-1 ).
| Lesion | Age | Sex | Distinguishing Clinical History | Distinguishing Clinical Presentation | Imaging Modality of Choice | Distinguishing Imaging Findings | Enhancement Pattern | Additional Findings |
|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma | 6th-7th decades | M = F | No specific symptoms, high level of suspicion is recommended in patients with long-standing disease, vague gastrointestinal symptoms, and weight loss | Abdominal pain (60%) Obstruction (40%) Gastrointestinal hemorrhage (24%) | Enteroclysis, CT enterography | Partial or complete small bowel obstruction or intussusception on plain film Barium studies: Apple core lesion, asymmetric wall thickening or infiltrative pattern More in proximal small bowel | Heterogeneous enhancement (hemorrhage, necrosis, or ulceration in 40% of cases) | Local extension, regional abdominal lymphadenopathy, distant metastases |
| Carcinoid | 6th-7th decades | M = F | Asymptomatic or present with carcinoid syndrome (cutaneous flushing, sweating, bronchospasm, abdominal pain, and diarrhea) | Classic carcinoid syndrome | Abdominal CT, CT enterography, nuclear scan | Intussusception on plain film Mesenteric mass on CT scan | Spiculated margins, low attenuation Mass with fat stranding and minimal enhancement Hypervascular liver metastases | “Spokewheel” or “sunburst” appearance on CT Encasement of mesenteric vessels leading to ischemia of affected bowel loops Octreoscan study is more sensitive in localizing carcinoids and metastatic disease compared with CT, MRI, or endoscopy. |
| Lymphoma | Bimodal, <10, >50 | M > F | Chronic anemia, weight loss | Abdominal pain, diarrhea, steatorrhea | Abdominal CT or CT enterography | Four patterns of involvement: (1) multiple nodules at multiple sites; (2) single large mass, triggering point for intussusception; (3) infiltrative pattern, presents as asymmetric wall thickening or aneurysmal dilatation of bowel loop; (4) exophytic mass | Low-attenuation soft tissue mass with minimal enhancement | Enlarged lymph nodes in the chest, abdomen, or pelvis Aneurysmal dilatation of the involved bowel loop without obstruction |
| GIST | 5th-6th decades | M > F | Incidental finding on imaging | Bleeding and abdominal pain; directly correlates with size of tumor | CT enterography or small bowel series | Intramural exophytic mass with or without internal calcifications and necrosis Submucosal lesion with smooth surface and acute angle | Circumscribed exophytic low-attenuation mass with heterogeneous enhancement depending on hemorrhage, necrosis | Liver metastases can be hypervascular. |
| Metastatic disease | After the 5th decade | M = F | History of a primary neoplasm, especially melanoma or gastrointestinal, pancreatic, or ovarian in origin | Gastrointestinal bleeding, increased abdominal pain, bowel obstruction in patients with known neoplasms | Abdominal CT | Diffuse or nodular peritoneal and intestinal wall thickening “Bull’s eye” appearance on barium studies Intussusception | Variable | Primary tumor and other metastatic disease |
Secondary Malignancies of the Small Bowel
Etiology
Metastatic disease to the bowel is more common than primary malignancies. Mechanisms of metastatic seeding to the small bowel include intraperitoneal seeding, direct extension along the fascia or mesenteric attachments, hematogenous spread, and lymphatic extension. The mechanism of spread determines the radiologic appearance. Intraperitoneal spread is the most common mechanism and usually occurs as a result of spread via ascitic fluid. The primary neoplasms are usually gastrointestinal in men and ovarian in women. Direct invasion of the small intestine is seen from primary colon, pancreas, biliary, ovarian, renal, and adrenal malignancies. Hematogenous spread of primary neoplasms to the small bowel is rare. Melanoma, lung, breast, kidney, and gynecologic cancers are the most common tumors with embolic spread to the small bowel.
Lymphatic dissemination to the small bowel plays a small role. A typical example is spread of cecal carcinoma to the terminal ileum by retrograde lymphatic flow after occlusion of the pericecal lymphatic vessels. Melanoma is the extraintestinal malignancy with the greatest predilection to bowel metastasis, and the small bowel is the most common part of the gastrointestinal tract to be affected by metastatic melanoma.
Clinical Presentation
The primary tumor is known in most cases, and patients present with nonspecific signs such as abdominal pain, weight loss, anemia, gastrointestinal bleeding, or obstruction. The interval between the diagnosis of malignancy and intestinal obstruction caused by the metastatic disease can vary widely. Intermittent obstruction and anemia can be caused by intussusception, with metastatic lesions serving as a lead point. It is important to consider the possibility of metastatic small bowel lesions in the setting of nonspecific abdominal complaints or chronic unexplained iron deficiency anemia in patients with known malignancies.
Pathophysiology
When the mechanism of spread is direct invasion, the localization of an involved small bowel segment depends on the primary tumor. The infiltration involves a larger segment of small bowel in patients with ovarian cancer but shorter segments in the case of primary colon carcinoma. Pancreatic tumors and hepatic flexure tumors infiltrate the duodenum, whereas cecal tumors invade the terminal ileum.
Peritoneal fluid has a continuous flow within the anatomic pathways of peritoneal recesses and mesenteric reflections. A primary neoplasm or metastatic lymph node can break into the peritoneal cavity and initiate the peritoneal spread. The most common sites for the lodging and growth of peritoneal spread are the pouch of Douglas, right paracolic gutter, superior aspect of the sigmoid mesocolon, and terminal portion of the mesentery in the right lower quadrant. The negative pressure under the diaphragm and increased capillary forces make the dome of the liver a common site for peritoneal deposits. Peritoneal deposits on serosal surfaces adhere through fibrinous exudation and may incite a desmoplastic response.
Pathology
Hematogenous metastases can be solitary or multiple and tend to be submucosal. The masses are usually on the antimesenteric border where the vasa recta end in a rich submucosal plexus and can demonstrate central ulceration because of their limited blood supply.
Lymphatic blockage by the primary tumor can cause retrograde lymphatic flow and spread of tumor cells into the small bowel with the terminal ileum and proximal jejunum being the most common sites of involvement.
Imaging
Radiography
Direct invasion from another primary tumor usually produces mucosal destruction and narrowing of the lumen without the shouldering of the margins, which is a characteristic of a primary neoplasm ( Figure 28-1 ). Chronic radiation changes need to be differentiated from secondary invasion. The duodenum is a common segment for secondary invasion and can be invaded by pancreatic, colon, renal, and adrenal tumors.

Peritoneal deposits may be seen as rounded protrusions toward the lumen of the small bowel. Discrete separation of ileal loops, often with a parallel configuration, angulated tethering of mucosal folds on their mesenteric border, and narrowing of loops are suggestive of peritoneal seeding associated with some desmoplastic reaction. Striking angulation and marked fixation of small bowel loops can be seen in primary cancers, causing a significant desmoplastic reaction, such as pancreatic or gastric carcinoma.
Multiple, round, polypoid nodules mostly seen along the antimesenteric border of the small bowel are the most common radiologic finding for hematogenous metastases, especially from a primary or malignant melanoma ( Figure 28-2 ). The metastatic nodules tend to ulcerate centrally, and, when the lesions are small, ulcerations appear as target lesions (“bull’s eye” lesions) with collection of barium at the central ulcers. These nodules can act as a lead point for intussusception. Larger masses may have large ulcers or cavitations outlined with barium and may have a mass effect on the surrounding small bowel segments. Breast cancer metastases are rare but described as spreading through the submucosa, causing multiple strictures and intestinal obstruction. Metastases from lung or renal cell carcinoma are usually seen as solid or multiple large mesenteric masses with frequent ulcerations.

Computed Tomography
Computed tomography (CT) can show the metastatic lesions in the small bowel wall, mesentery, peritoneal surfaces, and lymph nodes and therefore can give a better idea about the size and extent of the disease. CT also can depict the extension of disease to the surrounding organs. Another advantage of CT is its ability to identify the primary tumor ( Figure 28-3 ). Intestinal bowel wall or peritoneal thickening (either nodular or plaque-like), mesenteric or omental nodules, and/or fat stranding are the common CT findings in patients with metastases to small bowel segments ( Figure 28-4 ). Metastases from melanoma manifest as enhancing mural nodules or focal thickening of the intestinal wall ( Figure 28-5 ).



Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) can provide a sensitive and accurate depiction of tumor involving the peritoneum and bowel serosa. Fat-suppressed, gadolinium-enhanced MRI is preferred for detection of small peritoneal or mesenteric tumors. Peritoneal tumors are found to be more conspicuous on delayed MR images obtained 5 to 10 minutes after intravenous injection of gadolinium. Even small implants can be depicted on delayed, fat-suppressed, gadolinium-enhanced gradient echo images. Diffusion weighted imaging in combination with conventional MRI is more accurate in detecting peritoneal metastasis.
Ultrasonography
Ultrasonography can be sensitive for detection of serosal, and superficial neoplastic deposits when performed with a high-frequency probe. However, it is limited in evaluating deposits between the loops or along the posterior wall. The focal thickened wall of the small bowel shows the nonspecific “pseudo-kidney” sign, which can be seen in both benign and malignant diseases.
Positron Emission Tomography With Computed Tomography
Peritoneal metastatic deposits can demonstrate uptake of fluorodeoxyglucose (FDG), and PET/CT may be a useful diagnostic tool when peritoneal biopsy is either unavailable or inappropriate ( Figure 28-6 ). However, further studies are needed to better determine the role of FDG-PET for evaluation of peritoneal carcinomatosis.

Imaging Algorithm
SBFT is noninvasive but relatively insensitive for detection of small intramural deposits and is completely blind to extraintestinal disease.
Enteroclysis can detect small intestinal metastases earlier than CT, but it is an invasive test. With the advances of CT technology, new techniques such as CT enterography or CT enteroclysis have emerged and can evaluate both intestinal and extraintestinal disease ( Table 28-2 ). Therefore, CT is often used as an initial tool for evaluation of abdominal symptoms in patients with known cancer. In cases complicated with bowel obstruction, CT should be the diagnostic test of choice (see Figure 28-18 ).
- •
Target lesion (bull’s eye): Central ulceration of the hematogenous metastases, most common in the malignant melanoma metastases
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Enteroclysis is very accurate in detecting smaller lesions involving the small bowel wall | Cannot show mesenteric, peritoneal disease or primary tumor | Radiation enteritis |
| CT | Very accurate in the detection of relatively larger intestinal lesions and extraintestinal disease | Limited sensitivity in detection of small mucosal or intramural lesions | Undistended bowel |
| MRI | Slightly more sensitive in depiction of small peritoneal lesions | Limited experience, longer study time, less spatial resolution compared with CT |
Differential Diagnosis
Symptoms of metastatic disease to the small bowel are very nonspecific; therefore, the clinical differential diagnosis is very wide. Complications related to the treatment (irradiation or chemotherapy) of the primary cancer, bowel ischemia, infection, inflammation, paraneoplastic syndromes, primary gastrointestinal tumors, and other causes of abdominal pain must be considered.
The primary tumor is usually known and helps in determining the diagnosis. Adhesions, metastases, and radiation enteritis are considered in the differential diagnosis of small bowel obstruction in patients with known malignancies. Adhesions usually cause linear compression of the lumen with straight margins. Ileocecal metastatic disease may resemble Crohn’s disease. Endometriosis can mimic multiple, small hematogenous metastases. Abdominal tuberculosis can cause peritoneal thickening, mesenteric fat stranding, bowel wall thickening, and adenopathy mimicking metastatic disease. Radiologic differentiation from neoplastic disease may be difficult.
Treatment
Medical Treatment
Medical treatment is aimed specifically against the primary tumor and may consist of hormone therapy, chemotherapy, and targeted biological therapy. There is a large variability in the expected effectiveness of the treatment, as well as in the prognosis of patients. Obstruction may be relieved, and the symptoms may resolve after chemotherapy in patients with breast carcinoma.
Surgical Treatment
Surgical treatment of secondary intestinal malignancies aims to relieve the intestinal obstruction and/or control the metastatic disease. Radiologic demonstration of noninvolved small bowel segments significantly contributes to the achievement of both of these targets. An accurate preoperative understanding of the extent of the disease is crucial for surgical planning. The specific anticancer therapy is almost always considered after surgery.
- •
Knowing if small bowel metastases are present changes the staging, and their presence may cause complications such as intussusception, bleeding, or obstruction.
- •
Knowing the extent of disease is important for surgical planning and to decide between medical versus surgical treatment.
- •
Any complications related to metastatic disease should be considered.
- •
Abnormalities that may have a similar clinical presentation should be excluded.
Primary Malignancies of the Small Bowel
Etiology
The most common malignant primary small bowel neoplasms are small bowel adenocarcinoma, carcinoid, lymphoma, and gastrointestinal stromal tumors (GISTs). Small bowel adenocarcinoma is a rare neoplasm, and the most important risk factor in the development of adenocarcinoma is Crohn’s disease. Higher incidence of small bowel adenocarcinoma is also associated with adenomatous polyps, villous adenomas, familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, celiac sprue, cystic fibrosis, and peptic ulcer disease.
Carcinoid tumors originate from the diffuse endocrine system outside the pancreas and thyroid and most frequently occur in the gastrointestinal tract (66.9%), followed by the tracheobronchial system (24.5%). They are frequently associated with specific syndromes such as Zollinger-Ellison syndrome, multiple endocrine neoplasia type 1, carcinoid syndrome, or neurofibromatosis type 1.
The exact cause of small bowel lymphoma is unknown. Various predisposing factors have been postulated to be responsible for small bowel lymphoma. A few of the predisposing factors include celiac disease, previous extraintestinal lymphoma, immunosuppressed conditions such as human immunodeficiency virus infection and acquired immunodeficiency syndrome, long-standing systemic lupus erythematosus, Crohn’s disease, and postchemotherapy conditions.
GISTs are CD117-positive mesenchymal tumors thought to originate from interstitial cells of Cajal that are normally part of the autonomic nervous system of the gastrointestinal tract.
Prevalence and Epidemiology
Primary adenocarcinoma of the small intestine accounts for less than 1% of all primary gastrointestinal tumors, with an estimated annual incidence of 0.25 to 0.4 per 100,000 population. It predominantly affects the duodenum and jejunum in 42% and 43% of cases, respectively. The ileum is involved in less than 15% of the patients, except in Crohn’s disease. The peak incidence is in the sixth and seventh decades of life.
Carcinoid is the second most common small bowel malignancy, representing approximately 25% of all primary small bowel tumors, and most commonly affects the ileum. In the past 30 years there has been an increase in the incidence of carcinoid tumors, with 41.8% of gastrointestinal carcinoids occurring in the small intestine, followed by the rectum (27.4%), appendix (24.1%), and stomach (8.7%). The appendix was thought to be the most common location for gastrointestinal carcinoids, but several authors have noted a decreased incidence of appendiceal carcinoids. This observation is probably due to the decreasing rate of appendectomies related to the increasing accuracy of diagnosing inflammatory appendicitis preoperatively.
The mean age at the time of diagnosis for all carcinoids is 61.4 years, and the disease occurs equally in men and women. Synchronous or metachronous malignancies occur in 29% of patients with small intestinal carcinoids.
Small bowel lymphoma is the third most common small bowel malignancy, representing 10% to 15% of small bowel malignancies. It can involve any portion of the gastrointestinal tract and predominantly targets the lymphoid follicles. It is most common in the ileum, and most manifest as intermediate- to high-grade non-Hodgkin’s lymphoma; T-cell variants are more often associated with celiac disease. Mediterranean abdominal lymphoma is a variant associated with immunoproliferative small intestinal disease and consists of diffuse lymphomatous infiltration of mucosa and submucosa in long segments of the small intestine.
There is a slight male predominance with bimodal age distribution, with peaks in those younger than the age of 10 and older than the age of 50. Small bowel lymphoma is multifocal in 15% of cases. Increased incidence of small bowel lymphoma was reported among patients with celiac disease.
GIST is the fourth most common small bowel neoplasm. It constitutes 9% of all small bowel malignant tumors. GISTs rarely involve the duodenum. They occur in the fifth and sixth decades and are more common in males. It is difficult to distinguish benign from malignant GISTs based on radiographic appearance alone.
Clinical Presentation
There is a significant overlap among the clinical presentations of small bowel neoplasms. The clinical presentation and diagnosis of small bowel adenocarcinoma are usually delayed by 6 to 8 months primarily because small bowel carcinomas are not amenable to endoscopic examination when they are distal to the duodenum. Clinical presentation includes abdominal pain in 66%, obstruction in 40%, and gross intestinal hemorrhage in 24% of patients. Especially in patients with long-standing bowel diseases, malignancy should be considered.
Patients with carcinoid tumor can be completely asymptomatic or may present with carcinoid syndrome (cutaneous flushing, sweating, bronchospasm, abdominal pain, and diarrhea) in less than 10% of cases. The syndrome most commonly occurs in patients with ileal carcinoids and hepatic or retroperitoneal metastases.
Patients with small bowel lymphoma can present with chronic anemia, weight loss, fatigue, diarrhea, steatorrhea, or vague dull abdominal pain. A palpable mass can be present in one third of cases. Acute gastrointestinal bleeding is less common than in small bowel adenocarcinoma, but the risk for perforation is higher.
When small, GISTs can be incidental on medical imaging. As they increase in size, the most common manifesting symptom is gastrointestinal bleeding and abdominal pain. Less common symptoms are bowel obstruction and a palpable mass.
Pathophysiology
Small bowel adenocarcinoma occurs most frequently in the duodenum. Carcinoid tumors are submucosal, are more common in the ileum, and produce a characteristic mesenteric mass through lymphatic spread. Lymphoma of the small bowel could be either a primary small bowel tumor or a manifestation of lymphomatous disease. In cases with primary small bowel lymphoma there is less evidence of mediastinal or peripheral lymphadenopathy or splenomegaly. The mesenteric lymph node involvement is limited to the region of involved bowel. Lymphoma can occur anywhere in the gastrointestinal tract but is more common in the distal small bowel. GISTs are more common in the jejunum and ileum and can be bulky, causing mass effect on adjacent organs with ulceration or necrosis.
Pathology
Grossly, small bowel adenocarcinomas present as solitary, infiltrative, polypoid, or annular obstructing lesions. Single or multiple ulcers can be associated with an infiltrative pattern. Histologically, they are most commonly mucin-secreting adenocarcinomas.
On gross pathologic study, carcinoids are white, yellow, or gray firm nodules that rarely exceed 3.5 cm in the intestinal wall. They can typically manifest as multiple nodules (30% of cases), exophytic masses, or intramural masses. They may protrude into the lumen as polypoid nodules or classically manifest as infiltrative fibrous lesions. These are typically slow-growing tumors that may cause superficial ulcerations and hemorrhage. The metastatic deposits of carcinoid in the lymph nodes, mesentery, and liver vary in size and gross morphology. Extensive involvement of the subserosa and adjacent mesentery stimulates local release of serotonin, which is responsible for the development of desmoplastic reaction. Mesenteric arteries and veins located both near and far from the tumor may be thickened and may result in intestinal ischemia.
Small bowel lymphoma is confined to a small bowel segment with regional lymphadenopathy. There is no evidence of hepatic or splenic involvement except by direct tumor extension and mediastinal lymphadenopathy. The peripheral blood smear and bone marrow biopsy could be normal. Small bowel lymphomas are usually diffuse and poorly differentiated and a common site of non-Hodgkin’s lymphoma in children.
GISTs can manifest as exophytic large masses arising from the stromal layer with signs of central necrosis, hemorrhage, and ulceration. They can be characterized as benign, borderline, of low malignant potential, or malignant based on the pathologic appearance.
Imaging
The diagnosis of small bowel neoplasms can be challenging because these tumors are often small, infrequent, and difficult to detect radiographically. Currently, small bowel series and enteroclysis are used for evaluation of small bowel tumors in detecting small lesions. CT is now considered an important modality that helps in detection and staging of disease. Improvement in CT technology, including the introduction of multidetector-row CT (MDCT) scanners and advanced three-dimensional (3D) imaging capabilities have improved interest in using CT routinely to evaluate small bowel neoplasms and evaluate a source of gastrointestinal bleeding or suspected obstruction, especially if the small bowel series or enteroclysis was negative. MDCT enteroclysis has an overall accuracy of 84.7% for depiction of small bowel neoplasms. Gastroenterologists are routinely using wireless capsule endoscopy to capture video images of the small bowel, which is otherwise difficult to visualize by routine endoscopic procedures. These studies are gaining popularity in recent years and have complemented other radiographic modalities such as CT and MRI. It is routinely performed in patients with gastrointestinal hemorrhage of unknown cause, anemia of chronic disease, and an obvious mass on CT scan.
Radiography
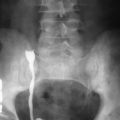
Plain films of the abdomen are helpful in evaluating partial or complete small bowel obstruction or intussusception as seen in cases of small bowel adenocarcinoma. Contrast radiographic studies are essential in the initial evaluation of small bowel diseases. Barium studies can demonstrate a wide spectrum of findings from “apple core” lesions, asymmetric wall thickening, to a more infiltrative process leading to a malignant stricture subsequently causing small bowel obstruction ( Figure 28-7 ).

Carcinoids can appear as trigger points leading to intussusception. Small bowel series are helpful in evaluating small polypoid and multifocal nodular appearances of carcinoid ( Figures 28-8 and 28-9 ). Ulcerations can be well appreciated on small bowel series as barium-filled craters on the surface of the lesion.


In evaluating small bowel lymphoma, small bowel series can show luminal narrowing of the involved segment with loss of mucosal pattern, thickening of the folds, and intraluminal filling defects, possibly with dilatation of the involved segment ( Figures 28-10 and 28-11 ). Small bowel obstruction is seldom seen. Nodular lesions can vary in size and are irregularly distributed. Widening of the lumen rather than narrowing is noted from circumferential involvement and destruction of the myenteric plexus leading to aneurysmal dilatation (see Figure 28-11 ).