Fig. 1
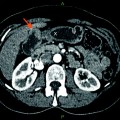
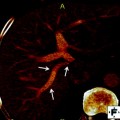
Uncovered (left) and covered (right) self-expandable metal stents (SEMS) (from first edition of book—needs permission)
SEMS have a number of advantages over plastic stents and are the recommended stent type for palliation of malignant biliary obstruction. First, they can expand up to three times the internal diameter of plastic stents. Consequently, they tend not to be obstructed by biofilm or bacterial overgrowth. Second, their duration of patency is 8–9 months, which is often longer than the life expectancy of patients with malignant biliary obstruction. Unlike plastic stents, if metal stents, which are constructed of an alloy mesh, become obstructed, it is usually tumor in-growth and overgrowth [24–26]. In an effort to combat this issue, SEMS with a membrane covering the alloy mesh were developed (covered SEMS). Studies have shown that covered SEMS do have a significantly longer patency, but are associated with increased risk of complications such as cholecystitis and pancreatitis as compared to uncovered SEMS [27].
In summary, endoscopically placed SEMS are the recommended first line of therapy for non-hilar malignant biliary obstructions. These stents offer a better quality of life for patients through the avoidance of additional procedures, increased symptom-free periods, potentially improved survival, and lower cost due to fewer endoscopies. Percutaneous drainage is the preferred approach for patients with hilar tumors, patients undergoing brachytherapy, or those who are not candidates for endoscopy.
Potential adjuvant therapies to stenting or bypass for palliation of symptomatic, unresectable biliary cancers include radiation and photodynamic therapy. The use of radiation has been shown to help with pain, stent patency, and survival (in the absence of metastatic disease) [28, 29]. Radiation therapy can be delivered as external beam radiation, brachytherapy, or a combination of the two. The majority of the studies showing a benefit of radiation used a combination of both modalities with patient survival ranging from 9 to 14 months [30–33]. Because radiation is often associated with higher rates of complications including cholangitis, longer hospital stays, and gastroduodenitis, it must be used selectively. Another approach to radiation therapy is radioembolization with Yttrium-90 microspheres. This approach is actually associated with fewer side effects and equivalent outcomes and is becoming the approach of choice in centers that offer this technology [34].
Another novel approach is photodynamic therapy. Photodynamic therapy involves the intravenous administration of a photosensitizing agent that is preferentially taken up by cancer cells. The cells are then exposed to light of specific wavelengths that actives the photosensitizer and kills the cells. The depth of tumor necrosis is 4–6 mm. Improvements in quality of life, biliary drainage, and survival have been reported with this approach [35–37]. Other regional therapy options for palliation also include transarterial chemoembolization (TACE) and radiofrequency ablation (RFA), which are discussed in other chapters of this text.
Symptoms associated with malignant biliary obstruction can include jaundice, pruritus, cholangitis, biliary colic, tender hepatomegaly, fatigue, weakness, anorexia, and cachexia. Previous studies have shown that relief of biliary obstruction can significantly improve the jaundice, pruritus, anorexia, indigestion, sleep and global health scores [38–41]. As patients with biliary cancers are also at increased risk for hepatic dysfunction, other sources of jaundice must also be investigated such as medications (including non-prescription), intrahepatic cholestasis, intraparenchymal tumor burden, and hemolysis.
2 Pruritus
One of the most frustrating symptoms of biliary obstruction and cholestasis is pruritus. If the cholestasis is due to biliary obstruction, successful relief of the obstruction is usually associated with complete relief of the pruritus. However, if the cholestasis is due to intrahepatic infiltration of tumor, pruritus can be extremely challenging to treat. Because this symptom impacts every aspect of a person’s life, management efforts are a priority for palliation. Despite significant research, the true cause of the pruritus remains unknown and the optimal management is not clearly defined. One prominent theory is that the pruritus of cholestasis is mediated centrally by endogenous opioids [42]. This theory has been supported by findings of increased opioidergic tone in animal models, as well as through clinical evidence of symptom relief from opioid antagonists such a naloxone, naltrexone, and nalmefene [43–49]. Administration of these agents must be used with caution due to the significant incidence of early and short-term opioid withdrawal-like symptoms including nausea, hallucinations, and dysphoria—even in opioid-naïve patients. Many of these side effects can be avoided with the initial use of ultra-low doses of intravenous naloxone before advancing to oral naltrexone for maintenance [50, 51]. Obviously, one concern is the prevalent use of opioid analgesics in biliary cancer patient. Fortunately, other studies in postoperative patients have shown that low-dose naloxone can improve the opioid side effects, including pruritus, without loss of the analgesic effects [52, 53].
Other potential options to treat pruritus of cholestasis include medications with central, opioid-related effects (e.g., GABA agonism by propofol), serotonin antagonists (e.g., ondansetron, mirtazapine, sertraline), intravenous lidocaine, and dronabinol. Other efforts have focused on removal of the pruritogens by such means as anion exchange resins (e.g., cholestyramine), plasmapheresis, and other extracorporeal techniques (e.g., hemodialysis). However, the use of such methods may not be feasible in patients with advanced cancers due to unpalatable side effects. Drugs that induce hepatic enzymes such as rifampin and phenobarbital have also had some success. Because there is no demonstrated role for histamines in the pruritus of cholestasis, it is not surprising that antihistamines have not proven hugely effective outside of the potential sedative effect. Topical therapies such as bathing with sodium bicarbonate can be helpful [54]. Consultation with a dermatologist to identify potential primary skin lesions and for topical medication recommendations is also advisable. Finally, in terminally ill patients with refractory pruritus, sedation may be helpful.
3 Gastric Outlet and Duodenal Obstruction
Due to their proximity to the distal stomach and duodenum, obstruction of the gastric outlet and/or duodenum is a common occurrence in patients with biliary cancer. Most patients present with nausea and vomiting of undigested food. Many will have upper abdominal distention and tympani. Imaging studies often reveal a distended stomach with retained enteric contents. Acute symptoms can be managed with a nasogastric tube, bowel rest, and intravenous resuscitation, including aggressive electrolyte repletion. For patients with chronic gastric outlet obstruction, special attention should be paid to chloride replacement which is lost in large quantities with emesis of gastric secretions. If significant weight loss is present or there may be a delay in definitive treatment, it may be reasonable to consider parenteral nutrition while planning additional palliative measures.
Options for managing these obstructions include intraluminal stenting, surgical bypass, and decompression gastrostomy with possible feeding jejunostomy. The potential benefits of stenting include immediate palliation of nausea and vomiting with a less invasive procedure than surgical bypass and earlier resumption of oral nutrition [55, 56]. Stents may be particularly useful in patients with advanced metastatic disease, who are poor surgical risk or who are technically inoperable. Flexible SEMS can be placed using endoscopic or fluoroscopic techniques. Stenting provides a comparable survival outcome with equivalent morbidity and mortality to surgical bypass [57]. In a systematic review of the literature comparing endoscopic stenting with open surgical bypass from 1990 to 2008, Ly et al. found that endoscopic stenting was more likely to result in tolerance of oral intake (OR 2.6; p = 0.002), in a shorter period of time (mean difference of 6.9 days, p < 0.001) and with a shorter hospitalization (mean difference of 11.8 days, p < 0.001) as compared to open surgical bypass. Similar findings were reported by Zheng et al. [58]. Based on these findings, it is also likely that stenting is less expensive than surgical bypass. The major limiting factor for the endoscopic approach is being unable to pass the scope through the obstruction. The major complications reported are gastric ulceration, bowel perforation, biliary obstruction, stent dysfunction, and stent migration. Stent placement would be contraindicated in patients with multiple sites of obstruction and should be considered carefully in patients with peritoneal carcinomatosis who are at risk for more distal obstructions.
For patients in whom stenting is not an option, surgical bypass can relieve both the symptoms of the obstruction and allow the patient to resume enteral nutrition. Surgical bypass, most commonly in the form of a gastroenterostomy, can either be performed laparoscopically or through a relatively small upper midline incision. The estimated risk of morbidity and mortality from these procedures is 25–60 and 0–25 %, respectively [57, 58]. While surgical bypass is usually technically successful, patient selection with regard to preoperative nutritional status and life expectancy is imperative to the palliative success of this approach. For example, in addition to general surgical risks such as bleeding or infection, a patient with chronic gastric outlet or duodenal obstruction who is malnourished is at increased risk for a leak from the intestinal anastomosis. Such a life-threatening complication could rob the patient of both time and any remaining quality of life. Other potential complications specific to gastric bypass include dumping syndrome, alkaline reflux gastritis, and delayed gastric emptying.
Placement of a gastrostomy tube for decompression is another option for palliation of gastric outlet, duodenal and non-operable small bowel obstruction, or profound gastrointestinal dysmotility from carcinomatosis. Gastrostomy tubes can be placed endoscopically, fluoroscopically, or surgically (either laparoscopic or open). Decompression gastrostomy tubes provide patients the ability to drain the stomach as needed for nausea and to avoid vomiting. It also allows them to drink liquids and eat some soft foods for pleasure and comfort. It does not allow for the enteric maintenance of nutrition. Many endoscopists, surgeons, and international radiologists are leery of placing gastrostomy tubes in the setting of malignant ascites. They are concerned about the risk of infecting the ascites, intraperitoneal leakage from the stomach due to poor apposition to the anterior abdominal wall, as well as leakage of ascites from around the tube. However, there is a growing body of literature demonstrating the safety of placing gastrostomy tubes in patients with malignant ascites from a variety of tumors [59–61]. Paracentesis prior to or concurrent with gastrostomy placement is advisable. Also, consideration of placing a peritoneal drainage catheter at the time of gastrostomy may also help lower any risk associated with the ascites. As gastrostomy may be the only viable palliative option for these patients, all efforts to manage the ascites and increase the safety of gastrostomy placement are warranted.
4 Pain
Pain is another symptom commonly experienced by patients with advanced biliary cancers. The type, location, and size of the biliary cancer will determine the intensity and quality of pain experienced. For example, a peripheral cholangiocarcinoma may only cause pain after growing to a size large enough to cause capsular stretching of the liver (hepatic distention syndrome), whereas a patient with gallbladder cancer may present with pain due to the presence of stones or an obstructed bile duct. Others may develop pain due to metastatic disease within the peritoneum. The pain can have a number of different qualities. The pain of hepatic distention is often described as a discomfort in the right subcostal region that may radiate to the right neck, shoulder, or scapula. This may be accompanied by acute subcostal pain often worsened by respiration. On the other hand, the pain of a bile duct obstruction may be more intense and colicky in nature. Regardless of the origin of the pain, relief is essential.
While a comprehensive review of cancer pain management is beyond the scope of this chapter, some guiding principles are provided. For patients with more refractory pain, or patients who are more difficult to palliate due to chronic opioid use or other medical comorbidities, either a pain service or palliative care consult is appropriate. However, for 80–90 % of patients, the approach proposed by the World Health Organization (WHO) analgesic ladder should be effective [62]. This simple, three-step ladder focuses on the intensity of the pain, rather than the etiology, as the main consideration in analgesic selection (Fig. 2). The goal is complete relief of the patient’s pain. For patients with mild cancer pain (1–3 on the numeric rating scale) (Table 1), the WHO analgesic ladder recommends starting with non-opioid analgesics (aspirin, ibuprofen, acetaminophen), which can be combined with adjuvant drugs if a specific indication exists (i.e., a tricyclic antidepressant in addition to acetaminophen for a neuropathic pain component). Caution is advised in using these medications in patients with advanced biliary or gastrointestinal cancers if there are any concerns for gastrointestinal bleeding or liver dysfunction. If non-opioid analgesics do not provide adequate relief or the pain worsens, then the patient moves to level two of the ladder and is considered to have moderate pain (4–6 on the numeric rating scale). These patients should be treated with opioids such as oxycodone, codeine, propoxyphene, or the opioid-like drug, tramadol. These opioids can be given as combination drugs with a non-opioid analgesic such as acetaminophen or separately but concurrently. The advantage of giving them separately is that the opioid dose can be increased beyond the dose-limiting maximum of the acetaminophen. These drugs can also be given concurrently with non-steroidal anti-inflammatory agents in patients without contraindications. Patients who present with severe pain (7–10 on numeric rating scale) or whose pain is not relieved by the appropriate administration of the drugs on the second step should receive an opioid used for moderate to severe pain (step three). The gold standard in this group is morphine. Other options include hydromorphone, fentanyl, methadone, diamorphine, phenazocine, levorphanol, and oxymorphone. These drugs can also be combined with non-opioid analgesics and other adjuvant agents.
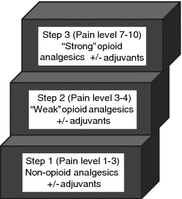

Fig. 2
Representation of World Health Organization analgesic ladder
Table 1
The World Health Organization (WHO) analgesic ladder to assess pain
Rating | Numeric rating for intensity of pain |
|---|---|
0 | No pain |
1–3 | Mild pain (nagging, annoying, interfering little with ADL*s) |
4–6 | Moderate pain (interferes significantly with ADLs) |
7–10 | Severe pain (disabling; unable to perform ADLs) |
Another important consideration in the management of pain in patient with advanced biliary cancer is the route of administration. Many of these patients may be approaching a transition in goals of care to hospice care, and the logistics of pain management need to be considered. Oral pain medications are the easiest to manage but may not be an option for patients with a gastric outlet or duodenal obstruction. Sublingual and transdermal formulations and rectal suppositories are other options for patients who cannot use oral medications. Subcutaneous administration is also effective and not particularly uncomfortable. Finally, patients on hospice can be set up at home with intravenous access and a patient-controlled analgesia (PCA) device.
Since relief of the patient’s pain is the goal, pain medications should be administered “around the clock” and the next dose should be given before the pain returns. By definition, waiting for the patient’s pain to return or increase in intensity does not achieve the goal of pain relief. Also, the appropriate dose of the medication is one that relieves the pain. It must be remembered that patients with liver failure may have a higher bioavailability and decreased clearance and therefore may require a lower dose than expected. Thus, it is advisable to start out at lower doses for these patients. Also, because opioids are largely renally excreted, patients with renal failure can be at risk for buildup of opioid metabolites. This can manifest as myoclonus and hypesthsias, which can be confused for worsening pain leading to administration of more opioid [63]. For patients in renal failure, fentanyl is the opioid of choice due to the buildup of fewer metabolites. All opioid-based pain regimens need to be accompanied by a bowel regimen containing a stool softener and laxative. Finally, all of these efforts should be combined with non-pharmacological methods of cancer pain where possible including radiotherapy, surgery, chemotherapy, hormone therapy, regional anesthetic techniques, physiotherapy, and psychological, cognitive, and spiritual approaches.
5 Nausea
Nausea is one of the most common and debilitating symptoms experienced by patients with advanced cancers. Patients with biliary cancer are at particularly high risk to suffer from nausea as both of the main brain centers involved in nausea and vomiting, the chemoreceptor trigger zone (CTZ) and vomiting center (VC), may be involved. It is important to have a basic understanding of these pathways in order to have the best chance to treat the nausea [64, 65]. The CTZ is located in the area postrema in the floor of the fourth ventricle, where there is essentially no blood–brain barrier. Chemoreceptors in this area are triggered by drugs, toxins, and metabolites in the systemic circulation. The VC is a diffuse interconnecting neural network that integrates emetogenic stimuli with parasympathetic and motor efferent activity to produce the vomiting reflex. In addition to receiving afferents from other regions of the brain and vestibular system, the VC receives afferents from the vagus and splanchnic nerves, the gastrointestinal tract (including chemo- and mechanoreceptors in the liver and gut), and peritoneum. The principle receptors in the CTZ are dopamine type 2 (D2). The principle receptors in the VC are muscarinic cholinergic (Achm) and histamine type 1 (H1). Both sites have serotonin type 3 (5HT3), and the VC also has serotonin type 2 (5HT2) [66]. Finally, neurokinin type 1 (NK1) receptors are concentrated in areas of the brainstem involved in emesis [67]. Understanding these pathways can guide antiemetic selection.
Before prescribing any medications for nausea, there are some non-pharmacologic efforts that may be beneficial. All efforts should be made to reduce any stimulus to nausea such as cooking smells, unpleasant odors, or strong scents. Small, appealing, bland meals and cool, carbonated drinks are also likely to be more palatable. To select an antiemetic, an effort should be made to identify the most likely cause of the nausea—i.e., a drug or toxin versus stretched mechanoreceptors in the stomach due to obstruction. This will help identify the most likely pathway—CTZ versus VC—and the most likely neurotransmitter receptor involved (Table 2). The most potent antagonist of the suspected receptor should then be selected and administered by a route that ensures that the drug will reach its target. The dose should be titrated, the response reviewed frequently, and the medication should be given regularly. If the symptoms persist, it is important to review the likely causes. If a new cause becomes apparent or if there are multiple causes identified, multiple agents may be necessary to target each receptor involved [68].
Table 2
Types of antiemetics used to relieve nausea
Common causes of nausea | Receptors | Site | Drug class | Drug example |
|---|---|---|---|---|
Opioid induced | D2 | CTZ | Butyrophenones | Haloperidol |
Gastric stasis | D2 | Intestine CTZ | Prokinetics | Metoclopramide, domperidone |
Intestinal obstruction/peritoneal irritation | D2 H1 ACHm | CTZ VC Intestine | Phenothiazines Antihistamines Anticholinergics | Prochlorperazine Diphenhydramine Hyoscine |
Chemotherapy/PONV | 5-HT3 | Intestine CTZ | 5-HT3 antagonists | Ondansetron |
Late-onset chemotherapy related | NK1 | Widespread | NK1 antagonist | Aprepitant |
In addition to chemical (chemotherapy, initiation, or escalation of opioids) and gastrointestinal causes of nausea (including severe constipation), other causes of nausea need to be considered in advanced cancer patients in order to direct therapy at the cause. These causes might include bowel obstruction, metabolic disturbances, or central nervous system metastases. In these circumstances, other therapies such as corticosteroids, radiation, or surgery may also be indicated. Anxiety-induced nausea and anticipatory nausea are also very common in cancer patients. Often, the patients or caregivers can identify anxiety as the trigger; however, if not, other sources must be ruled out before ascribing the nausea to anxiety. Psychotherapeutic techniques to address the anxiety combined with a long-acting benzodiazepine for a short period of time can be helpful [69]. The addition of an antidepressant with secondary anxiolytic properties may also be helpful. Anticipatory emesis is a conditioned response in patients who have suffered nausea and vomiting with cytotoxic agents. Any memory can trigger it. Psychotherapy combined with alprazolam has been shown to be helpful [70].
6 Cancer Cachexia and Fatigue
Perhaps one of the most emotionally distressing symptoms of advanced cancer is cachexia. The latest working definition of cancer cachexia is “a multifactorial syndrome defined by a negative protein and energy balance driven by a variable combination of a reduced food intake and abnormal metabolism. A key defining feature is ongoing loss of skeletal muscle mass, which cannot be fully reversed by conventional nutritional support, leading to progressive functional impairment” [71]. Cancer cachexia is diagnosed by involuntary weight loss greater than 10 % over a 6-month period with ongoing weight loss in the previous 1–2 months. It is important to recognize that in some patients such as those who are obese, have large tumors, or have significant fluid retention, muscle mass may be lost without loss of weight. More recently, it is recognized that cachexia is a spectrum ranging from preclinical cachexia (patients with cancer who manifest early clinical signs of cachexia without significant weight loss) to patients with late (irreversible) cachexia (advanced muscle wasting, a low performance status, and life expectancy of less than three months) [72]. Identifying cachexia in its preclinical stage may allow providers to intervene earlier and delay its progression.
Clinical assessment of a patient for cancer cachexia should include (1) evaluating for anorexia and decreased food intake; (2) determining the presence of a catabolic drive caused by progressive disease and inflammation; (3) identifying decreased muscle mass and reduced strength; and (4) reduced physical, psychosocial, and social function [71]. There are both primary and secondary causes of cachexia. The pathogenesis of primary anorexia–cachexia is a combination of decreased food intake due to neurohormonal (brain–gut axis) eating dysregulation (anorexia), catabolic inflammation (muscle-liver axis), and anabolic dysbalance with muscle loss (brain-muscle axis) [73–75]. Secondary anorexia and secondary cachexia can also be divided into three groups: (1) starvation due to impaired oral intake or malabsorption (secondary anorexia); (2) loss of muscle mass (i.e., due to prolonged immobility or protein loss (due to comorbidities such as nephritic syndrome or repeat paracenteses) (secondary cachexia); and (3) a catabolic state and comorbidities associated with cachexia (chronic infection, poorly controlled diabetes, hyperthyroidism) [71].
Optimal management of cancer cachexia is still evolving. The best therapeutic option for treating cancer cachexia is treatment of the underlying disease [76




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree