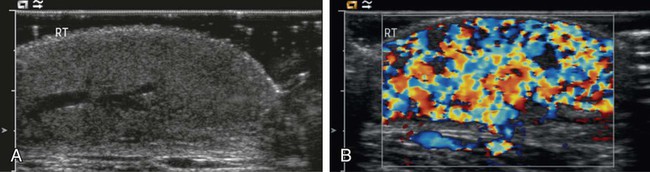
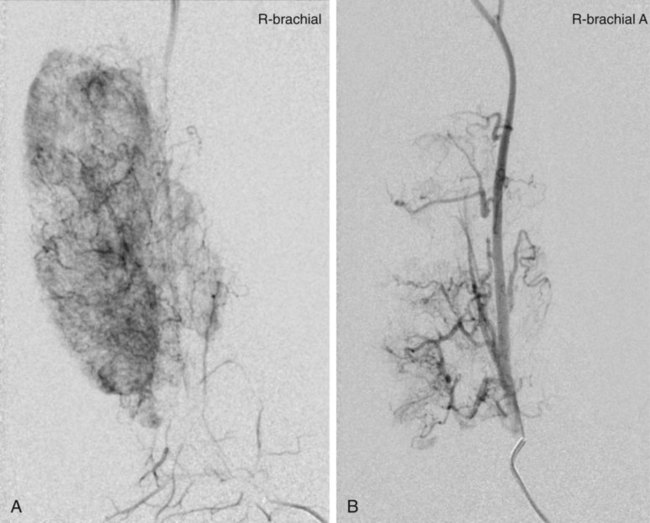
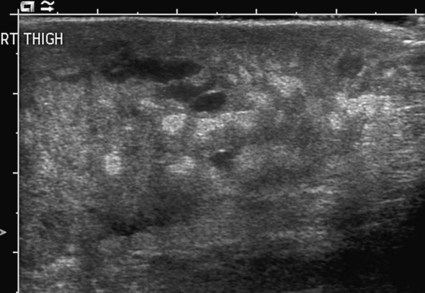
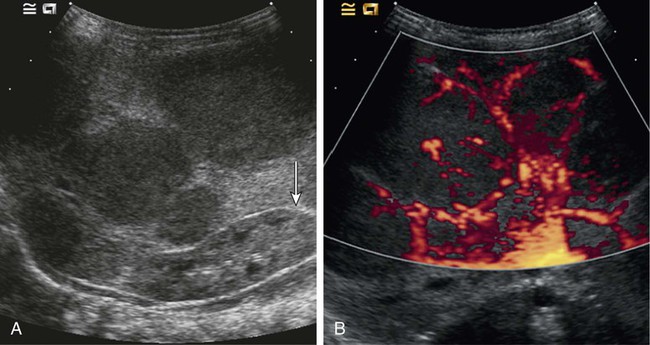
Vascular anomalies are often poorly managed for a number of reasons: they are uncommon (other than the true infantile hemangioma), their mode of presentation is extremely variable, their classification has been very confusing and is still poorly understood by the majority of doctors, and their treatment is challenging. The interested interventional radiologist is in an ideal position to play a major, if not the lead, role in patient management. So many aspects of assessment and treatment require radiologic input, and it is essential that this involvement starts at the time of referral and the patient’s first visit to the outpatient clinic. It is not good practice for a radiologist to accept a referral for endovascular treatment of a vascular malformation without first assessing the patient.1 In 1982, Mulliken and Glowacki proposed what was at that time a very new way of classifying vascular anomalies.2 Their system has been modified very little since then, with only minor changes made by the International Society for the Study of Vascular Anomalies (ISSVA) when it was established in 1992. This is now the most widely recognized system in use, encouraging management based on underlying histology and natural history of the lesion subtypes. The dichotomous Mulliken and Glowacki classification separates vascular tumors from vascular malformations. The vascular tumor arm includes the common infantile hemangiomas as well as rarer congenital hemangiomas and other vascular tumors, many of which are high flow. The vascular malformation arm comprises low-flow venous and lymphatic malformations and high-flow arteriovenous malformations (AVMs). Infantile hemangiomas are by far the most common lesions in this subgroup. They are not present at birth, appearing at around 2 to 4 months of age. Typically they demonstrate a highly active period of growth and proliferation and then slowly involute, disappearing during school age. It is this natural history that clearly separates them from vascular malformations that, in contrast, are present at birth and grow commensurately with the child. Infantile hemangiomas are usually superficial lesions with a characteristic bright red color and are easily diagnosed clinically, rarely troubling the radiologist. Occasionally, deeper lesions occur that do not exhibit skin staining. Such rapidly growing masses generate consternation and urgent referral. The diagnosis is simple because the imaging features are usually characteristic. Ultrasound demonstrates a well-defined echogenic lesion that is highly vascular with large central feeding arteries and draining veins (Fig. 45-1). The highly arterialized nature of the lesion simply reflects its extremely active proliferation. Occasionally, biopsy may be required to allay concerns. Infantile hemangiomas express glucose transporter-1 (GLUT-1), a simple diagnostic marker. Core needle biopsy, when necessary to exclude more serious pathology, is usually straightforward under ultrasound guidance because lesions are typically superficial. A 16G coaxial technique is advocated in view of the highly vascular nature of these lesions, allowing a single pass through the tumor capsule and plugging of the biopsy track with Gelfoam (Pfizer, New York, N.Y.) pellets; complications in the authors’ experience are rare. Perhaps surprisingly, most of these highly vascular tumors do not cause vascular steal or cardiac compromise and can be managed conservatively. Intervention is indicated when lesions cause significant mass effect or disfigurement. Subglottic lesions and those that obstruct the visual axis are of particular concern. Pharmacologic management is now the mainstay of treatment in this group, with β-blockers the preferred first-line therapy.3 Novel therapies such as the use of rapamycin to suppress stem cell proliferation are also evolving.4 Occasionally, high-output cardiac failure occurs in children with very large infantile hemangiomas, particularly those involving the liver, and this requires urgent intervention. Particle embolization of the capillary bed is a simple and effective approach, aiming to reduce the vascularity of the lesion and stabilize the child’s cardiovascular status until the natural history of the lesion causes involution (Fig. 45-2). Surgical ligation or coil embolization of the main feeding artery usually fails, the highly angiogenic tumor simply recruiting other arterial feeders. Particle embolization may also be indicated to reduce tissue bulk in rare instances where large lesions cause platelet sequestration and clotting factor consumption, although there is currently no published evidence for this. Congenital hemangiomas form two distinct clinical subgroups defined by their natural history. Some involute very rapidly in the first few months of life and are termed rapidly involuting congenital hemangioma (RICH), and others never involute (noninvoluting congenital hemangioma, NICH). Both types are histologically and immunophenotypically distinct from infantile hemangiomas.5 Neither expresses GLUT-1. Biopsy of such lesions is sometimes helpful to allow clinicians to predict outcome. Urgent embolization of very large congenital hemangiomas is often requested but should be avoided if possible, because a proportion of these lesions will involute quickly without intervention. Infantile and congenital hemangiomas make up the vast majority of lesions in the vascular tumor arm of the Mulliken and Glowacki classification of vascular anomalies, but it is important to recognize other much less common lesions. These include the tufted angioma (TA), kaposiform hemangioendothelioma (KHE), hemangiopericytoma, and pyogenic granuloma. Recognition of TAs and KHEs in particular is important for two reasons: they often have a more unusual aggressive appearance despite their benign histology (Fig. 45-3), precipitating direct referral to oncologists, and they are sometimes associated with the Kasabach-Merritt phenomenon (KMP).6 Both are GLUT-1 negative on biopsy and have characteristic histology, so biopsy is often useful. Kasabach-Merritt phenomenon describes a pattern of variable but often severe coagulopathy and thrombocytopenia; it is seen in association with a variety of soft-tissue lesions, but most commonly KHE. Children with vascular tumors who exhibit KMP show a variable response to therapy, and mortality rates are high. Evolving pharmacologic approaches to other vascular tumors may have a role to play in the management of these lesions. Embolization remains an alternative approach in an attempt to reduce tissue bulk and downregulate the drivers for KMP, but evidence is lacking. Both infantile and congenital hemangiomas occur in the liver in a proportion of children. The infantile lesions are almost always multifocal or diffuse and associated with multiple cutaneous hemangiomas, whereas the congenital lesions are typically solitary. Ultrasound confirms their high-flow nature and can demonstrate shunting at an intralesional level; this simply reflects disorganized neovascularity within these rapidly proliferating lesions (Fig. 45-4). It is important that these are not labeled as AVMs because this inevitably leads to high levels of clinical anxiety and the potential for unnecessary intervention. Infantile hemangiomas of the liver have the same natural history as the more common skin lesions, showing slow involution over time. Hepatic congenital hemangiomas are more commonly the RICH subtypes rather than NICH, and involute spontaneously and quickly. As elsewhere, biopsy is diagnostic and should guide management. Additionally, serum α-fetoprotein (AFP) should be measured to exclude hepatoblastoma. Because serum AFP concentrations vary widely between individuals in the neonatal period and can be elevated in children with liver hemangiomas, serial measurements are recommended to ensure levels fall rapidly in the first few weeks after birth. Extensive liver hemangiomas can cause dramatic hepatic enlargement in the neonate, leading to respiratory and cardiac compromise. Thrombocytopenia and coagulopathy can also be seen. In such instances, medical management is often successful.7 In refractory or fulminant cases, embolization may buy the child some time by reducing lesion size and the volume of blood flow through it. A combination of coil and particle embolization is usually successful. Left axillary arterial access is often a useful approach in the neonate,8 and carbon dioxide angiography minimizes the volume of fluid and iodinated contrast media required in these hemodynamically unstable children.
Management of High-Flow Vascular Anomalies
Introduction
Classification
Vascular Tumors
Infantile Hemangioma
Congenital Hemangioma
Other Vascular Tumors
Liver Vascular Tumors in Childhood
Management of High-Flow Vascular Anomalies