High-risk breast lesions (HRLs) are a group of heterogeneous lesions that can be associated with a synchronous or adjacent breast cancer and that confer an elevated lifetime risk of breast cancer. Management of HRLs after core needle biopsy may include close imaging and clinical follow-up or excisional biopsy to evaluate for cancer. This article reviews histologic features and clinical presentation of each of the HRLs, current evidence with regard to management, and guidelines from the American Society of Breast Surgeons and National Comprehensive Cancer Network. In addition, imaging surveillance and risk-reduction strategies for women with HRLs are discussed.
Key points
- •
High-risk breast lesions are a group of heterogeneous lesions that can be associated with a synchronous or adjacent breast cancer and that confer an increased lifetime risk of breast cancer.
- •
Surgical excision is typically recommended for atypical ductal hyperplasia (ADH), but small-volume ADH that is completely removed at core biopsy may be surveilled rather than excised in certain scenarios.
- •
Observation after risk assessment and multidisciplinary input may be appropriate for pure flat epithelial atypia, lobular neoplasia with imaging-pathologic concordance and adequate sampling, papillomas without atypia, and small radial scars.
- •
For women with a history of ADH or lobular hyperplasia and greater than 20% lifetime risk, clinical breast examination with risk-reduction counseling every 6 to 12 months and annual screening mammogram are recommended, in addition to consideration of annual breast MR imaging and chemoprevention.
Introduction
More than 10% of breast biopsies performed based on findings at mammography yield high-risk lesions (HRLs). HRLs refer to a group of heterogeneous lesions that can be associated with a synchronous or adjacent breast cancer and that confer an elevated lifetime risk of breast cancer. , These lesions include atypical ductal hyperplasia (ADH), flat epithelial atypia (FEA), lobular hyperplasia (which refers to atypical lobular hyperplasia [ALH] and lobular carcinoma in situ [LCIS]), papillomas, and radial scars.
There are no universally agreed-on recommendations for the management of HRLs, which is thought to be due to variations in reported upgrade rates, limitations in study designs of available literature (eg, single-institution retrospective studies and selection bias with regard to which patients undergo surgical excision), lack of radiology-pathology concordance, and lack of consensus among pathologists regarding the diagnostic criteria for HRLs. , This lack of consensus has led to variations in patient care, with management after core needle biopsy including close imaging and clinical follow-up or excisional biopsy to evaluate for ductal carcinoma in situ (DCIS) and invasive cancer. This article reviews histologic features and clinical presentation of each of the HRLs, current evidence with regard to management, and guidelines from the American Society of Breast Surgeons (ASBrS) and National Comprehensive Cancer Network (NCCN). In addition, imaging surveillance and risk-reduction strategies for women with HRLs are discussed.
High-risk lesions
Atypical Ductal Hyperplasia
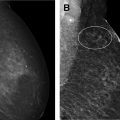
ADH is characterized by the intraductal proliferation of uniform epithelial cells with monomorphic round nuclei that fill part of the involved duct or completely fill the duct but measure less than 2 mm or involve fewer than 2 ducts ( Fig. 1 ). ADH resembles low-grade DCIS with cytologic and architectural atypia but is of limited extent. ADH typically presents in asymptomatic women as calcifications at mammography and less commonly as a mass at sonography ( Fig. 2 ). It can also be seen as non-mass enhancement at MR imaging.


A meta-analysis of 93 studies found that the pooled upgrade rate of ADH to DCIS or invasive cancer was 29% (95% confidence interval, 26%–32%) for lesions that were surgically excised and 5% (95% confidence interval, 4%–8%) for lesions that were surveilled. The invasive cancer upgrade rate was 9% (95% confidence interval, 7%–11%) for lesions that were surgically excised and 3.4% (95% confidence interval, 1.8%–6.4%) for lesions that were surveilled. Risk factors associated with upgrade risk include age at biopsy, breast cancer risk, lesion size, presence of multiple lesions, needle gauge, and number of biopsies.
Given the relatively high upgrade rate of ADH to cancer, and the challenge at pathology of distinguishing ADH from DCIS, the ASBrS and NCCN recommend surgical excision for most ADH. , However, per the ASBrS guidelines, small-volume ADH that is completely removed at core biopsy may be observed if the imaging findings and pathology results are concordant and after consideration of breast cancer risk factors and input of the multidisciplinary team. Several models to predict upgrade risk of ADH have been developed, which can inform the decision-making process. , One institution proposed the following guidelines for surveillance rather than surgical excision of ADH: lesion smaller than 6 mm with complete removal of calcifications, smaller than 6 mm with incomplete removal of calcifications but 2 or fewer foci of ADH, or 6 to 21 mm with 2 or fewer foci of ADH. , Of 41 excised cases that met criteria for surveillance, only 1 was upgraded at surgery, for an upgrade rate of 2%.
Flat Epithelial Atypia
FEA is characterized by the intraductal proliferation of epithelial cells with low-grade cytologic atypia, which demonstrate a “flat” pattern of growth along the ductal or acinar wall with no architecturally atypical structures. The involved terminal duct-lobular units (TDLUs) are enlarged and dilated ( Fig. 3 ). ADH and DCIS demonstrate both cytologic and architectural atypia, whereas FEA is characterized by the presence of cytologic atypia without architectural atypia. FEA typically presents in asymptomatic women as calcifications at mammography, often grouped in distribution and amorphous in morphology ( Fig. 4 ). Less commonly, FEA can present as an irregular mass at sonography.


In a meta-analysis of 32 studies, the upgrade rate of FEA to cancer at surgery was widely variable, ranging from 0% to 42%; the overall pooled upgrade rate estimate was 11.1%. Among studies of higher quality, the overall pooled upgrade rate estimate was 7.5% (95% confidence interval, 5.4%–10.4%), and the invasive cancer upgrade rate was only 3% (95% confidence interval, 1.9%–4.5%). Risk factors associated with an increased cancer upgrade risk include a genetic mutation, the presence of concurrent ADH, ALH, or radial scar, and incomplete removal of the imaging finding at core needle biopsy. In the aforementioned meta-analysis, the upgrade rate of FEA to ADH at surgery was also widely variable, ranging from 0% to 60%; the pooled upgrade rate estimate was 17.9% overall and 18.6% among studies of higher quality.
Given the overall low cancer upgrade rate of FEA, and that upgraded cases are more often DCIS than invasive cancer, the ASBrS recommends observation with imaging and clinical follow-up of pure FEA. Surgical excision is recommended for FEA with concurrent ADH. Guidelines from the NCCN also indicate that pure FEA may not require surgical excision, but specific criteria for surveillance versus surgical excision are not provided. Although the ASBrS and NCCN favor surveillance of pure FEA, surgical excision may be warranted to evaluate for a higher-risk lesion (eg, ADH) to inform decision-making with regard to chemoprevention.
Lobular Hyperplasia
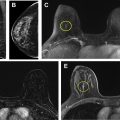
The term lobular hyperplasia refers to ALH and LCIS. ALH and LCIS are characterized by the proliferation of dyshesive monomorphic epithelial cells, often with intracytoplasmic vacuoles, that arise within the TDLUs and distend the acini. ALH is less extensive than LCIS, with ALH defined as involving less than 50% of the acini in a TDLU ( Figs. 5 and 6 ). , Pleomorphic LCIS and florid LCIS are morphologic variants of LCIS: pleomorphic LCIS has high-grade cytologic features, and florid refers to an architectural growth pattern causing expansion of ducts and lobules. ALH and LCIS are thought to represent incidental findings at core needle biopsy that are typically associated with calcifications ( Fig. 7 ). ADH and LCIS can also be seen as non-mass enhancement or as an enhancing mass at MR imaging.


Several published studies report low overall upgrade rates of ALH and classic LCIS. For example, of 85 consecutive cases in which core needle biopsy yielded pure lobular neoplasia, upgrade rates to cancer were 3% (95% confidence interval, 0%–9%) for radiologic-pathologic concordant cases and 38% (95% confidence interval, 9%–76%) for discordant cases. Among 77 patients with pure lobular neoplasia, the upgrade rate to cancer was only 1% when reviewed by central pathology. Among 68 patients with pure lobular neoplasia, no upgrades at surgery were observed in normal-risk patients with calcifications identified at mammographic screening. Features associated with increased upgrade risk include radiologic-pathologic discordance, extensive LCIS with more than 4 foci, and imaging for high-risk indications. ,
According to guidelines from the ASBrS, observation of ALH and LCIS can be offered in the setting of small-volume lesions, radiologic-pathologic concordance, and the absence of other HRLs. Per guidelines from the NCCN, options for management of ALH and LCIS with radiologic-pathologic concordance include observation with imaging and clinical follow-up or surgical excision. The increased risk of upgrade associated with multifocal/extensive LCIS involving more than 4 TDLUs is noted in the NCCN guidelines. Surgical excision of higher-risk LCIS variants (ie, pleomorphic and florid LCIS) is widely recommended ( Fig. 8 ). , ,

Papilloma
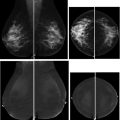
Intraductal papillomas are characterized by fibrovascular cores lined by 2 cell types, an inner myoepithelial layer and an outer epithelial layer ( Fig. 9 ). Central papillomas, which are the most common type of papilloma, originate in the large ducts and are usually solitary, whereas peripheral papillomas arise in the TDLUs and can be multiple. Papillomas can present with nipple discharge or as a palpable mass or can be discovered incidentally at imaging. On imaging, papillomas manifest as a mass or calcifications at mammography, an intraluminal filling detect or duct dilatation at galactography, an intraductal mass at sonography, or an enhancing mass at MR imaging ( Fig. 10 ).