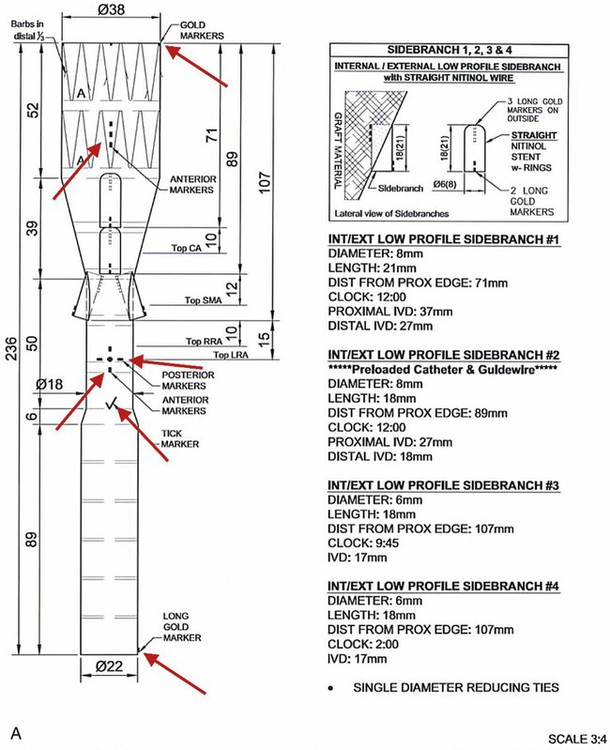
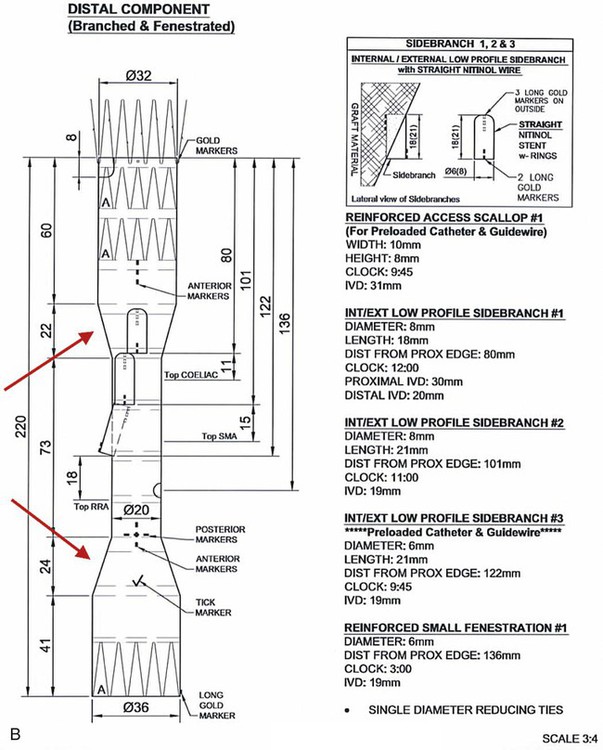
Chapter 49 Mark R. Tyrrell, C. Jason Wilkins and Stéphan Haulon The majority of patients who have infrarenal aortoiliac aneurysms can now be relatively safely treated, but the prognosis for patients who have large (>6 cm diameter) aortic aneurysms that involve the origins of the abdominal visceral vessels remains grave. The untreated natural history is only 17% survival at 5 years,1 and the annual combined rupture/dissection/mortality rate is 14%.2 Although the first successful open repair was reported 56 years ago,3 the risks of treatment remain considerable. In the pre-endovascular era, Crawford’s considerable experience showed that operative mortality risk and the risk of paraplegia relates to aneurysm extent, leading to the Crawford classification.4 This classification (now slightly modified)5 still forms the foundation underpinning the technical approach and risk assessment of TAAA repair. Even in the “best risk” subgroup (Crawford type IV TAAA), the physiologic demands of open surgical repair place considerable stresses on patients that are likely to be beyond the reserve of many, and the recovery time is long in survivors. Endovascular infrarenal aortic aneurysm (AAA) repair results in significantly lower 30-day mortality than anatomically equivalent open surgery.6–8 Similarly, endovascular repair of isolated thoracic aortic aneurysms (TEVAR) is associated with a lower risk of death than its conventional equivalent.9–11 Since the first generations of devices available for endovascular aneurysm repair (EVAR) were relatively simple tubes or bifurcated grafts, initial attempts to extend the benefits of EVAR to patients with TAAA led to “hybrid” solutions. These involve laparotomy, extra-anatomic bypass to the visceral vessels, and then extended EVAR to exclude the aneurysmal segment while preserving blood flow to the vital organs. Although initial reports were greeted with enthusiasm,12–14 good results have not been universal,15,16 and the approach does not exploit all of the potential advantages of a “pure” endovascular approach (e.g., lesser surgical insult, better physiologic control, rapid recovery). This unmet need, together with rapid technologic advances, has encouraged the development of more ambitious endovascular solutions to extend the principles and potential benefits of EVAR to the challenging TAAA patient group. Total endovascular repair of a true aortic aneurysm using a branched device was first described in 2001.17 To emphasize the point, FEVAR is a technique used in the proximalization of sealing zones for AAA with short aortic “necks”; BEVAR is used specifically in the management of aneurysms where the visceral bearing segment is dilated and unsuitable for use as a sealing zone—the thoracoabdominal aortic aneurysms (TAAAs). In many situations, instances could be applied as evidenced by the approaches of either Chuter (BEVAR only)18 or Bicknell (FEVAR only).19 1. Before anesthesia, it is important to check the device against the printed plan and description. 2. The chosen access vessels are cannulated by surgical cutdown and percutaneous access as required. In practice, the most common routes are surgical exposure of one femoral artery (the side associated with the larger diameter and least tortuosity), percutaneous puncture of the contralateral femoral artery, and open exposure of the left distal subclavian, axillary, or brachial. 3. Full anticoagulation is established using sodium heparin. This is monitored, corrected, and maintained until arterial closure is complete (target activated clotting time 300 seconds). 4. If necessary, proximal (tube) extension devices are passed via the iliac system and abdominal aorta and are deployed to seal at the planned proximal landing zone. 5. The main body is prepared, oriented correctly (using the various check markers and other radiopaque markers integral to the device) (Fig. 49-1, A), and passed to the planned proximal sealing zone (aorta, proximal TEVAR extension device, or previously placed elephant trunk, according to the case), reoriented if necessary, deployed, and released from the delivery system, which is then withdrawn. We have found the use of a centimeter-marked pigtail catheter is useful in positioning the device accurately—the objective being to “land” with the branch ends 15 to 20 mm superior to (or inferior to in the case of upward-pointing branches) the target vessel. 6. Distal aortic extension devices (tube or bifurcated according to the requirements of each case) are then introduced and deployed to provide a distal seal. All sealing zones are molded using a compliant balloon. 7. All large sheaths occupying the iliac system are removed, and the femoral arteriotomy is closed over a small access sheath to reperfuse the pelvis and lower limbs. 8. At this point, the aorta is effectively relined, the proximal and distal aortic landing zones are sealed, the pelvis and legs have normal blood supply, but the aneurysm sac and its visceral branches are still pressurized and perfused via the open device branches. 9. The use of a “through-and-through” wire is an essential adjunct. This can be achieved by cannulation of the endograft via the upper limb access, passage of a fine 300-cm-long wire, and snaring and retrieving it via the femoral access. This allows a degree of tension and aids passage of the long sheath (10F, 80 cm) inserted from the upper limb access vessel, which might otherwise tend to collapse and coil into the aortic arch. 10. Each branch and target vessel is accessed, stented, balloon-molded, and angiographically checked as a complete sequence before the next vessel is attempted (Fig. 49-2
Management of Thoracoabdominal Aneurysms by Branched Endograft Technology
Introduction
Philosophy
Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Management of Thoracoabdominal Aneurysms by Branched Endograft Technology