You’re about to sign off on a negative screening mammogram when her history catches your eye. She’s only 32 years old. Her sister was just diagnosed with breast cancer at age 40. Two of her paternal aunts have also had breast cancer. She should probably get mammograms every year, but does she also need a screening magnetic resonance imaging (MRI)? What if she were 72 years old with a similar family history?
It used to be so easy. Every woman age 40 and older got a mammogram every year, and that was it. If a woman had a mother or sister with breast cancer at a young age, then you could start screening with mammography early. We had little else to offer these patients, and the radiologist’s role was much more limited.
Radiologists are now playing an increasingly active role in assessing breast cancer risk and making recommendations regarding risk reduction strategies. It can be very rewarding to empower women who are at high risk for breast cancer to manage their risk.
We have all experienced the worried well—women who are convinced that they will be diagnosed with breast cancer. About 28% of average-risk women overestimate their risk. Unfortunately, 57% of women who are actually high risk underestimate their personal risk of breast cancer. Although some gynecologists and other health care providers are very knowledgeable, radiologists are often considered the experts on this topic. This is not to say that radiologists will start running breast cancer risk clinics. However, we do see large numbers of women for mammographic screening and routinely collect much of the history needed to estimate risk. We are therefore in a great position to identify women who may need to have a more significant conversation about their breast cancer risk and to recommend the steps that can be taken to manage it.
The Three Categories of Breast Cancer Risk
Breast cancer risk factors can be divided into three categories: personal, breast-related, and genetic risk factors ( Table 13-1 ). The pillars are not equal.
| PERSONAL | BREAST | GENETIC |
|---|---|---|
|
|
|
Personal Risk Factors
These are the best known risk factors. We tend to ask women most of these questions prior to mammography. Although we know these well, no single personal risk factor increases lifetime risk to even a moderate level.
The personal risk factors are largely related to hormone exposure. Women who start menstruating at a young age or complete menopause at an older age have a greater duration of exposure to estrogen and progesterone and increased risk. Childbearing results in maturation of the lobules, so early parity is associated with lower breast cancer risk, and nulliparity and late parity are associated with a higher risk.
Women using menopausal hormone therapy (MHT) with estrogen alone (usually with hysterectomy) are not at elevated risk for breast cancer, but the use of estrogen with progesterone increases breast cancer risk by about 1.7 times. The risk occurs even in the first few years, so there is no “safe” period under which MHT can be used without considering breast cancer risk. That said, many women in perimenopause would rather cut off their right arm than not use hormone therapy. The symptoms of menopause can be severe. Sleep disruption and mood disorders make these women (and sometimes their families and friends) desperate. Women must find a balance between breast cancer risk and a reasonable lifestyle. Newer MHT regimens using lower doses and combining estrogens with a selective estrogen receptor modulator may reduce the associated breast cancer risk.
Fatty tissue contains an enzyme (aromatase) that converts circulating steroids to estrogens. Obesity is associated with a 30% to 50% increase in breast cancer risk in postmenopausal women. A 20-lb weight gain after age 18 is associated with a 50% to 100% increase in breast cancer risk. Sadly, the incidence of obesity in the United States has doubled since the 1960s. Although we mammographers are often accused of inflating the incidence of breast cancer, the incidence has also increased in women who are not engaged in screening. Certainly part of this may be related to the increasing incidence of obesity.
Alcohol consumption also increases breast cancer risk. Every 10 g of alcohol consumed per day (about one drink) increases breast cancer risk by 9%. This is likely mediated through interference with liver metabolism of estrogen products.
Breast-Related Risk Factors
A breast biopsy showing one of the high-risk lesions (atypical ductal hyperplasia [ADH], atypical lobular hyperplasia [ALH], lobular carcinoma in situ [LCIS]) is associated with an increase in lifetime risk of breast cancer. LCIS and ALH together are considered lobular neoplasia, which is associated with a 7- to 10-fold increase in breast cancer risk. If there is also a family history of breast cancer, then the risk associated with LCIS is doubled. A biopsy showing ADH is associated with a 3- to 4-fold increase in breast cancer risk.
Breast density is a moderate risk factor for breast cancer independent of other risk factors including age, parity, and family history. Women with very dense breast tissue have a 4-fold increase in breast cancer risk compared with women whose breast tissue is fatty-replaced. The risk is dose-dependent; compared with women with fatty breasts (relative risk [RR] 1.0), women who have scattered, heterogeneous, and extremely dense breast tissue have a 2.2-, 3.4-, and 5.3-fold increase in breast cancer risk. The reason some women have dense tissue and others do not is not known, but may reflect hormone metabolism, collagen deposition patterns, or growth hormones. At least 30% of breast density is genetically mediated.
Women who have had mantle radiation therapy to the chest, usually for treatment of lymphoma, are at high risk. The risk is increased about 5-fold compared to average-risk women. The younger the age of exposure, the higher the subsequent risk of breast cancer. The increased risk of breast cancer begins about 7 to 8 years and peaks at about 15 years after the radiation exposure. For patients who received 20 Gy or more to the chest/mediastinum, the Children’s Oncology Group recommends annual mammography and MRI beginning at age 25 or 8 years after the exposure, whichever is later. Mantle radiation is being phased out—most women who receive radiation therapy for Hodgkin disease now undergo a more graded radiation dose across the chest, resulting in a lower dose to the breast tissue. It remains to be seen if this may lower breast cancer risk enough to forgo MRI screening.
Genetic Risk Factors
Family history of breast cancer increases risk. If a woman has one primary relative (mother, sister, daughter, father, brother, son) with breast cancer, her lifetime risk is 13% compared to 8% for women without a first-degree relative with breast cancer. She becomes high risk (lifetime risk 21%) when two first-degree relatives have breast cancer.
There are several genetic mutations that increase the risk of being diagnosed with breast cancer. The most common mutations are in the BRCA1 and BRCA2 genes, but there are other less common mutations that are also associated with increased risk.
All of the genes that are associated with breast cancer risk are DNA repair (tumor suppressor) genes. We all have a little bit of damage to our DNA on a daily basis. Maybe you sat in the sun during lunch and an ultraviolet ray hit the DNA in your skin cells. Your body repairs these little hits using tumor suppressor genes. When these genes, like the BRCA1 gene, for example, have a mutation and don’t work so well, the person carrying that mutated gene has a higher likelihood of developing breast and other cancers. The types of cancers depend upon the mutation type and gene affected.
We often call women “ BRCA carriers” but in reality they are “ BRCA mutation carriers.” These genetic mutations actually occur with equal frequency in both men and women and are autosomal dominant. They are not located on the X or Y chromosome. Each of us has two sets of DNA, one inherited from our father and one from our mother. Therefore, if Dad has a BRCA2 mutation, you have a 50 : 50 chance of inheriting that copy of his gene. If you do get that gene, you will inherit an increased risk for cancer. Incidentally, if Dad is a BRCA2 mutation carrier, he is at increased risk for cancer of the pancreas, prostate, colon, and, yes, breast ( Boxes 13-1 and 13-2 ).
- •
Genetic testing is recommended when the risk of mutation is at least 10%.
- •
Mutation testing is separate for each gene ( BRCA , Li-Fraumeni, Cowden).
- •
The relative with breast cancer ideally should be tested first.
- •
If the patient has a primary relative who is a known genetic mutation carrier, her negative genetic test is considered a true negative. For example, Mom is a known BRCA2 mutation carrier. If your patient tests negative, then she did not inherit that gene. She is not high risk and can have routine screening.
- •
It’s not a perfect test. A negative genetic test does not change her high-risk status if there is not a known mutation in the family.
Personal history of breast cancer (BC) and at least one of these:
- •
Diagnosed ≤ age 45
- •
Diagnosed ≤age 50 and:
- •
Close relative with BC < age 50 OR
- •
Close relative with ovarian cancer (any age)
- •
- •
Two breast cancers , one ≤ age 50
- •
Two close relatives with BC at any age
- •
Two close relatives with pancreatic cancer
- •
Ashkenazi Jewish ancestry
First- or second- degree relative (not cousin) with breast cancer who was:
- •
Diagnosed ≤ age 45
- •
Diagnosed < age 50 and:
- •
Close relative with BC < age 50 OR
- •
Close relative with ovarian cancer (any age)
- •
- •
Diagnosed with two BCs, one ≤ age 50
- •
Diagnosed at any age and has two close relatives with breast or ovarian cancer, any age
- •
Diagnosed at any age and has two close relatives with pancreatic cancer, any age
Primary relative with known genetic mutation but not yet tested.
Breast cancer in a close male family member
Ashkenazi Jewish ancestry and a first- or second-degree relative with BC
Note: Close relative (first-, second-, third-degree relative): mother, sister, half-sister, daughter, aunt, grandmother, cousin, brother, father, uncle.
Hereditary breast and ovarian cancer (HBOC) syndrome is a term applied to carriers of mutations in either BRCA1 or BRCA2 . Alterations occur on chromosome 17q in BRCA1 and 13q in BRCA2 . These mutations are much more common in the Ashkenazi Jewish population (Eastern European Jewish ancestry). Most of the Jewish population in the United States is of Ashkenazi descent. About 2% of those of Ashkenazi Jewish descent carry a mutation of BRCA1 or BRCA2 compared with about 0.5% of the United States population overall.
The lifetime risk of breast cancer is highest for women with a BRCA1 mutation. These women have a 55% to 85% lifetime risk of breast cancer; BRCA2 mutation is associated with a 25% to 60% lifetime risk of breast cancer. Mutation carriers of BRCA1 and BRCA2 also differ in the incidence of other cancers. BRCA1 mutation carriers are at very high risk for ovarian cancer in women (21 times more likely than for average-risk women!), testicular cancer in men, and stomach, renal, bladder, liver, gallbladder, and pancreatic cancer in both sexes. BRCA2 mutation carriers are at elevated risk for ovarian cancer in women, prostate cancer in men, and stomach, liver, gallbladder, and pancreatic cancer in both sexes.
Li-Fraumeni syndrome is associated with alteration of chromosome 17p. These mutation carriers have a 60% to 90% lifetime risk of breast cancer. These men and women also have an increased risk of rare cancers, including sarcomas, brain cancer, leukemia, and adrenal cancer.
Cowden syndrome is associated with alterations in chromosome 10q. These mutation carriers have a lifetime risk of breast cancer of 30% to 50%. Benign thyroid disease and thyroid cancers are common in these families. Multiple hamartomas occur on mucosal surfaces. Lipomas are also common.
Bannayan-Riley-Ruvalcaba syndrome is a rare disorder associated with developmental disorders that are apparent at a young age.
Neurofibromatosis (NF1) is also likely associated with an increase in breast cancer risk. It may be associated with moderate risk rather than the high risk due to the preceding genetic syndromes.
Breast Cancer Risk Models
We know that women who are genetic mutation carriers are at high lifetime risk for breast cancer, but what about women with a combination of risk factors? For example, what if a woman has a mother with breast cancer and she had a biopsy showing LCIS? Breast cancer risk models combine risk factor information to yield a woman’s lifetime and either 5- or 10-year risk of developing breast cancer ( Table 13-2 ).
| RISK LEVEL | LIFETIME RISK | ASSOCIATED RISK FACTORS |
|---|---|---|
| Average risk | <15% | |
| Moderate risk | 15-20% | LCIS, ADH, ALH |
| Dense breast tissue | ||
| Intermediate family history | ||
| Previous breast cancer | ||
| High risk | >20% | Hereditary breast and ovarian cancer (HBOC) syndrome (e.g., BRCA ) |
| Other genetic mutations | ||
| Chest irradiation at a young age | ||
| Combination of risk factors |
Gail Model
The Gail model is the oldest and best validated breast cancer risk model. It was developed using data from the Breast Cancer Detection Demonstration Project (BCDDP), which was conducted in the 1980s. This model primarily focuses on personal risk factors. It also includes biopsy showing ADH and family history of breast cancer in primary relatives (mother, sister, daughter).
This model is short and quick. It is only seven questions. Unfortunately, the accuracy of the Gail model is only about 50%. This model was developed before the BRCA mutations were discovered and does not include the paternal side of the family. It is the only model validated in African-American women.
Claus, BODICEA, and BRCAPro Models
Claus, BODICEA, and BRCAPro models target identification of genetic mutation carriers. These models do not include personal or breast-related risk factors. These models are based on family pedigree, including both maternal and paternal history. These models are great for identifying women with a strong family history of breast cancer. The last two models also predict the risk of a woman carrying a BRCA mutation. Genetic counseling and testing are recommended for women who have at least a 10% risk of being a BRCA mutation carrier. The accuracy of these models for predicting breast cancer risk in the general population is only about 56% ( Box 13-3 ).
- •
Consider using a breast cancer risk assessment model in your practice. These models are often included in automated breast imaging reporting systems and are also available online and as smartphone apps.
- •
Follow the National Comprehensive Cancer Network (NCCN) Guidelines for identifying women who are considered likely to be high risk. These guidelines are summarized in Box 13-2 .
- •
Recommend risk reduction strategies for women who may be at high risk. This, of course, includes breast MRI, but remember that there are many other tools to reduce risk. You might phrase your recommendation in your report as something like, “This patient may be at high risk for breast cancer. She may benefit from risk reduction strategies, including screening breast MRI.”
- •
Reach out to regional primary care providers, including gynecologists, to develop a regional strategy for managing high-risk women that will include genetic counseling/testing.
- •
Consider developing a high-risk clinic . Ours is held 1 day per week and run jointly by a breast surgeon and gynecologic oncologist. The latter’s surgical volume doubled within 1 year due to oophorectomies for prophylaxis or abnormal imaging appearance. This was a win-win project for our patients and the institution.
Tyrer-Cuzick Model
The Tyrer-Cuzick model is the most comprehensive, including personal risk factors, biopsy showing ADH or LCIS, and a family cancer pedigree. This model is more time intensive, but readily available. The accuracy is about 80%.
Breast Density Is Missing from the Models
When breast density is added to a comprehensive risk model, risk is slightly overestimated. Several investigators, including Dr. Harvey, are working on the development of a comprehensive risk assessment model that includes breast density.
Breast Cancer Risk Reduction
Reducing the risk of breast cancer includes both steps that make it less likely that a woman will develop the disease as well as screening efforts that allow earlier detection, with the goal of reducing the mortality rate.
All women can reduce their risk of developing breast cancer by limiting alcohol consumption (including red wine…sorry!), breastfeeding, controlling weight, exercising regularly, and not using MHT. Women age 40 and younger who spend at least 4 hours per week exercising reduce breast cancer risk by 60%. The effect is not as strong for postmenopausal women. Women ages 50 to 65 years who partake in moderate-to-strenuous exercise reduce breast cancer risk by 30%. Screening mammography reduces breast cancer mortality rate rather than incidence.
Options for risk reduction for women who are at high risk (>20% lifetime risk) include intensive screening, chemoprevention, and prophylactic surgery. Intensive screening may result in earlier detection and a reduction in breast cancer mortality rate. Chemoprevention with tamoxifen or raloxifene reduces breast cancer incidence by 50% to 60%, but primarily for ER/PR (estrogen receptor/progesterone receptor) positive cancers. Reduction in mortality rate may or may not be significant. Prophylactic mastectomy reduces breast cancer incidence by about 98%.
Let’s remember that these women are at risk for many other cancers besides breast cancer. Offering screening MRI is the first step, but these patients need much more. Many women will opt for prophylactic oophorectomies. Screening with colonoscopy is often also recommended.
Screening Breast MRI
Screening breast MRI is indicated for women who have at least a 20% to 25% lifetime risk of breast cancer. The vast majority of women with this level of risk are known or suspected genetic mutation carriers, although a combination of risk factors such as a biopsy showing LCIS and a primary relative with breast cancer may result in a similar lifetime risk.
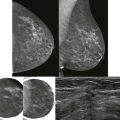
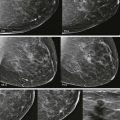
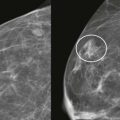
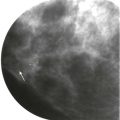
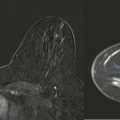
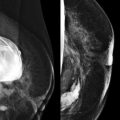
Screening breast MRI detects about 30 cancers per 1000 women screened. This is a lot of cancers! For comparison, mammography finds 3 to 8 cancers per 1000 average-risk women screened. There are no trials that study mortality rates for screening breast MRI, but the majority of cancers detected are small invasive cancers that are node negative. The mean size of invasive cancers detected on screening MRI is 0.7 to 2.0 cm, and 65% to 100% are node negative ( Fig. 13-1 ). This implies that many of the cancers detected by MRI are likely found at a lifesaving point in time.