Mediastinal masses are relatively uncommon and include a wide variety of abnormalities of neoplastic, congenital, vascular, and lymphatic etiology that most radiologists encounter infrequently. Imaging plays a critical role in the identification and evaluation of mediastinal lesions, facilitating the formulation of focused differential diagnoses and guiding clinical management. In some cases mediastinal masses demonstrate imaging characteristics that can suggest a specific diagnosis; in other instances the imaging findings alone are inconclusive, and a combination of radiologic and clinical information is necessary to determine the next step in management.
Division of the mediastinum into specific compartments has traditionally served as a helpful tool for the identification and characterization of mediastinal masses and other abnormalities. Several different classification systems have been developed and used by anatomists, surgeons, and radiologists. The most commonly used scheme in clinical practice is the Shields classification system, whereas the traditional Fraser and Paré, Felson, Heitzman, Zylak, and Whitten models have been used to varying degrees in radiologic practice. One model has been proposed in an attempt to bridge the gap between the various schemes. Fundamental differences in the methods of mediastinal compartmentalization and terminology used in these systems have caused confusion and resulted in miscommunication among health care providers evaluating patients with mediastinal masses.
The existing models typically used in radiologic practice represent arbitrary divisions of the chest based primarily on the lateral chest radiograph that are nonanatomic. A classification scheme based on cross-sectional imaging techniques, principally computed tomography (CT) but also magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG)–positron emission tomography (PET)-CT, is necessary as these modalities are typically used to evaluate and diagnose mediastinal abnormalities, and a growing number of lesions are detected with CT studies performed for lung cancer screening, cardiac screening, and other purposes. In 2014 the Japanese Association for Research on the Thymus (JART) developed a four-compartment model based on CT, which was derived from a retrospective study of 445 nonconsecutive pathology-proven mediastinal masses. Based on discussions with experts in the field of mediastinal diseases, the International Thymic Malignancy Interest Group (ITMIG) has modified the JART model and introduced a new definition of mediastinal compartments to be used with cross-sectional imaging that has been accepted as a new standard.
In this chapter the new ITMIG mediastinal compartment classification system based on cross-sectional imaging is presented along with detailed information regarding the wide variety of masses and other abnormalities that may be encountered in the mediastinum and their predominant manifestations on imaging studies.
International Thymic Malignancy Interest Group Definition of Mediastinal Compartments
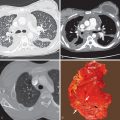
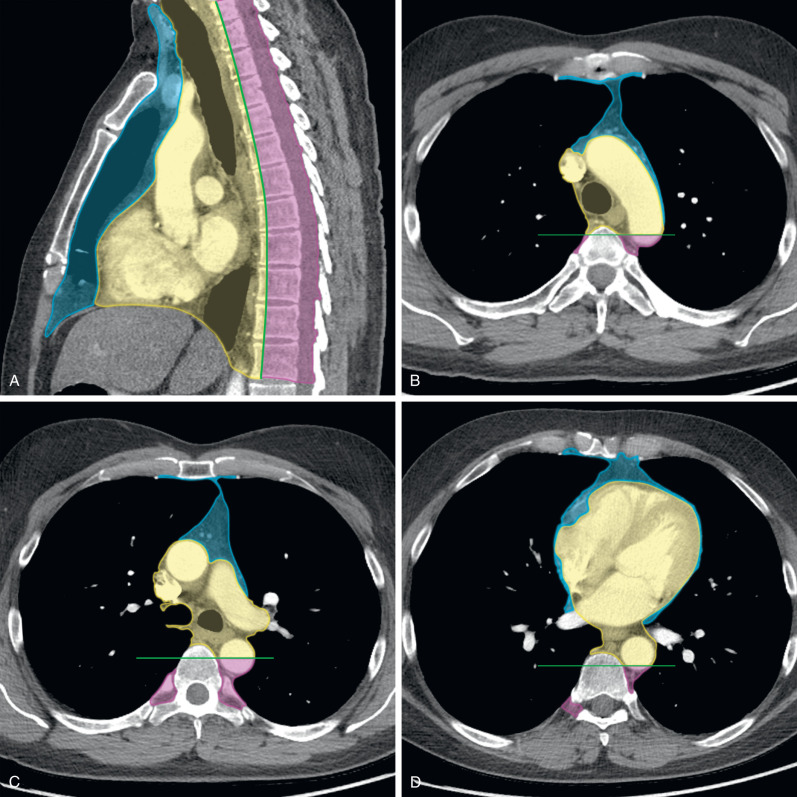
The ITMIG classification includes specific prevascular (anterior), visceral (middle), and paravertebral (posterior) compartments ( Table 77.1 ) and compartment boundaries, and the anatomic structures they contain can be readily identified on cross-sectional imaging ( Fig. 77.1 ).
| Compartment | Boundaries | Major Contents |
|---|---|---|
| Prevascular |
|
|
| Visceral |
|
|
| Paravertebral |
|
|
Prevascular Compartment
The boundaries of the prevascular compartment include (1) superiorly: the thoracic inlet, (2) inferiorly: the diaphragm, (3) anteriorly: the posterior border/cortex of the sternum, (4) laterally: the parietal mediastinal pleura, and (5) posteriorly: the anterior aspect of the pericardium (see Table 77.1 ). This compartment contains the thymus, fat, lymph nodes, and the left brachiocephalic vein. Therefore the most common abnormalities encountered in the prevascular compartment include thymic lesions (cysts, hyperplasia, and thymic epithelial neoplasms, such as thymoma, thymic carcinoma, and thymic carcinoid), germ cell neoplasms, lymphoma, metastatic disease, and intrathoracic goiter.
Visceral Compartment
The boundaries of the visceral compartment include (1) superiorly: the thoracic inlet, (2) inferiorly: the diaphragm, (3) anteriorly: the posterior boundaries of the prevascular compartment, and (4) posteriorly: a vertical line connecting a point on the thoracic vertebral bodies 1 cm posterior to the anterior margin of the spine, referred to as the visceral–paravertebral compartment boundary line (see Table 77.1 ). This compartment includes vascular structures such as the heart, superior vena cava (SVC), thoracic aorta, intrapericardial pulmonary arteries, and the thoracic duct, and nonvascular structures, including the trachea, carina, esophagus, and lymph nodes. It should be noted that the ITMIG model includes all structures enclosed by the pericardium in the visceral compartment. The extrapericardial pulmonary arteries and veins are considered pulmonary structures and are not included in this compartment. Lymphadenopathy caused by lymphoma or metastatic disease, duplication cysts, esophageal lesions, and cardiovascular abnormalities are responsible for most abnormalities that arise from the visceral compartment.
Paravertebral Compartment
The boundaries of the paravertebral compartment include (1) superiorly: the thoracic inlet, (2) inferiorly: the diaphragm, (3) anteriorly: the posterior boundaries of the visceral compartment, and (4) posterolaterally: a vertical line along the posterior margin of the chest wall at the lateral aspect of the transverse processes (see Table 77.1 ). This compartment contains the thoracic spine and paravertebral soft tissues. Therefore neurogenic neoplasms that arise from the dorsal root ganglia/neurons adjacent to the intervertebral foramina comprise most lesions in the paravertebral compartment. Other abnormalities that may be encountered include infectious (diskitis/osteomyelitis) or traumatic (hematoma) lesions, as well as miscellaneous conditions, such as extramedullary hematopoiesis.
Imaging of Mediastinal Masses
General Considerations
Greater than half of all mediastinal masses arise from the anterior/prevascular compartment, whereas one-fourth each are identified in the middle/visceral and posterior/paravertebral mediastinal compartments. In many instances localization and characterization of a mediastinal abnormality using CT are sufficient to make the diagnosis. In other cases correlation between imaging findings and clinical context, as well as additional imaging examinations, such as MRI and PET-CT, and histologic sampling through image-guided or surgical biopsy, is necessary to make a definitive diagnosis and guide further management.
Radiography
Chest radiography is the most commonly performed imaging examination and is often the first modality demonstrating findings suggestive of mediastinal pathology. Small lesions may not be visible or result in subtle abnormalities of mediastinal lines, stripes, and interfaces. For instance, a thymic epithelial neoplasm in the anterior mediastinum may only result in thickening of the anterior junction line. Therefore close evaluation of these landmarks is necessary on radiography. The lateral chest radiograph can be useful in identifying abnormalities that may not be visible on the posteroanterior radiograph, usually those that are only visible in the retrosternal space or overlying the upper thoracic spine. Large lesions can manifest in myriad ways, including more extensive irregularity of normal mediastinal contours or interfaces or one or multiple soft tissue masses.
Multiple radiographic signs have been described that can be useful in alerting the radiologist that a mediastinal mass may be present. One of these is the silhouette sign, which describes the loss of normal borders of intrathoracic structures and can assist the radiologist in identifying various mediastinal abnormalities. For instance, a mass in the right anterior mediastinum may obscure the right heart border, whereas a mass in the posterior mediastinal compartment may result in loss of the normal paraspinal line. The hilum overlay sign may help differentiate a mediastinal lesion from other abnormalities, such as cardiomegaly or enlarged pulmonary vessels.
Computed Tomography
CT is the imaging modality of choice for identifying, localizing, and characterizing mediastinal masses, and imaging features often enable the radiologist to formulate a focused differential diagnosis based on imaging alone. Tomiyama and colleagues evaluated 127 mediastinal masses of various etiologies in the prevascular compartment and demonstrated that CT was equal or superior to MRI in correctly diagnosing most of the lesions, apart from thymic cysts.
Specific information regarding mediastinal masses that should be noted on CT and reported include (1) location, size, and configuration; (2) density, heterogeneity, and enhancement; (3) fat, cystic components, soft tissue, and calcification; and (4) connection with or invasion of adjacent structures. Some of these findings are more diagnostically relevant than others. For instance, the presence of fat in a prevascular mediastinal mass on CT is highly suggestive of a small number of lesions, such as mature teratoma, thymolipoma, and others, whereas calcifications are nonspecific and may be associated with benign or malignant lesions.
Magnetic Resonance Imaging
MRI is rarely the first imaging modality used to evaluate chest symptomatology or abnormalities identified on chest radiography but is the most effective modality for distinguishing cystic from solid lesions (e.g., thymic cysts from solid tumors), identifying cystic and/or necrotic components within solid masses, identifying septations and/or soft tissue within cystic lesions, and distinguishing thymic hyperplasia and normal thymus from solid neoplasms. Unenhanced MRI with specific fluid-sensitive sequences can be performed to evaluate mediastinal masses and identify vascular involvement when patients are unable to receive intravenous (IV) contrast for CT examinations because of severe allergy and/or impaired renal function. Finally, chemical shift MRI using in-phase and out-of-phase sequences is the technique of choice for diagnosing thymic hyperplasia and distinguishing it from thymoma and other thymic neoplasms, as the former is characterized by the loss of signal intensity on out-of-phase imaging resulting from the presence of microscopic fat interspersed between thymic tissue.
Fluorodeoxyglucose–Positron Emission Tomography–Computed Tomography
The role of FDG–PET-CT in the evaluation of mediastinal masses is controversial, and several studies have been performed to investigate its efficacy in differentiating benign from malignant lesions and among specific types of malignant tumors. Kubota and colleagues showed that malignant mediastinal neoplasms demonstrated significantly greater FDG uptake than benign lesions using a standardized uptake value (SUVmax) equivalent cutoff of 3.5. Others investigations have used higher SUVmax thresholds, and the authors have suggested that PET-CT is complementary to conventional imaging modalities such as CT but noted that histologic sampling is required to confirm PET-CT findings. Luzzi and colleagues reported significant overlap between the SUVmax of FDG-avid malignant neoplasms, including high-risk thymic epithelial neoplasms (World Health Organization [WHO] types B2 and B3), lymphoma, paraganglioma, and nonseminomatous germ cell tumors (NSGCTs). Thymic epithelial neoplasms tend to exhibit variable FDG uptake on PET-CT, with some lesions demonstrating little to no FDG uptake. Sung and colleagues suggested that PET-CT could be used to distinguish low-risk thymomas (WHO types A, AB, and B1) from thymic carcinoma, and other investigators have reported that PET-CT could distinguish low-risk thymomas from high-risk thymomas (WHO types B2 and B3) and thymic carcinoma. However, other studies have been less definitive, with PET-CT not demonstrating clear benefit in staging.
Localization of Mediastinal Masses
Although localization of mediastinal masses to specific compartments is critical, precise identification of the compartment of origin may be difficult in some instances. For instance, a large lesion may involve multiple compartments or extend from one compartment to another, making the delineation of the precise site of origin challenging. The ITMIG has defined and recommends the use of two specific tools that can help in lesion localization: (1) the “center method” and (2) the “structure displacement tool.” The center method states that the center of a lesion, which is defined as its center point on the axial CT image on which the mass has the greatest diameter, localizes the mass to a specific compartment. This tool was used in the JART study and resulted in the accurate localization of all 445 mediastinal lesions. The structure displacement tool is particularly helpful when large mediastinal lesions displace organs from other mediastinal compartments. For example, a very large prevascular mediastinal mass may result in the posterior displacement of visceral compartment structures, such as the trachea or heart.
Imaging of the Prevascular Compartment
Thymic Epithelial Neoplasms
Thymic epithelial neoplasms are malignant tumors that affect 0.13 per 100,000 individuals in the United States and are the most common nonlymphomatous primary neoplasms of the prevascular/anterior mediastinum. The most common thymic epithelial neoplasms include thymoma, thymic carcinoma, and thymic neuroendocrine tumors.
Thymoma
Thymoma is the most common thymic epithelial neoplasm and primary malignancy of the prevascular mediastinum, with 1 to 5 cases per million individuals per year in the United States. The incidence is higher in Asians and African Americans compared with Hispanic and Caucasian populations, and men and women are affected equally. The incidence of thymoma increases with advancing age such that patients older than 40 years are most commonly affected, although the incidence starts to decrease after the age of 60 years. Thymomas are typically slow-growing neoplasms but may demonstrate aggressive behavior, such as invasion of adjacent structures and involvement of the pleura and pericardium; however, distant metastases are rare.
Symptomatic patients typically report clinical symptoms related to local effects of the primary tumor, including dysphagia, diaphragmatic paralysis, or SVC syndrome resulting from compression and invasion of adjacent structures. Approximately one-third of affected patients report chest pain, dyspnea, or cough. Systemic symptoms and paraneoplastic syndromes resulting from the release of hormones, antibodies, and cytokines by the neoplasm may be reported. The most common paraneoplastic syndrome associated with thymoma is myasthenia gravis. Although 30% to 50% of patients with thymoma exhibit clinical signs and symptoms of myasthenia gravis, only 10% to 15% of patients with myasthenia gravis have thymoma. Other associated paraneoplastic syndromes include hypogammaglobulinemia and pure red cell aplasia, which are present in 10% and 5% of cases, respectively. Several autoimmune disorders have been described in association with thymoma, including systemic lupus erythematosus, polymyositis, and myocarditis.
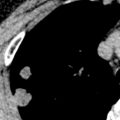
CT is the imaging modality of choice to evaluate thymoma and can help differentiate it from other prevascular masses. Thymoma typically manifests as a soft tissue mass with smooth or lobulated margins measuring 1 to 10 cm that characteristically arises from one lobe of the thymus ( Fig. 77.2 ), although involvement of the bilateral prevascular mediastinum has been described. After the administration of IV contrast, thymoma typically demonstrates homogeneous enhancement, although heterogeneity is present in approximately one-third of patients resulting from necrosis, cystic change, and/or hemorrhage. Several patterns of calcification have been observed, including punctate, linear along the capsule, and within the lesion. Thymomas may invade adjacent vessels, resulting in direct signs, such as irregularity of the vessel lumen contour, enhancement or obliteration of vessels, and endoluminal soft tissue. Other findings on CT, such as extensive mediastinal lymphadenopathy, pleural effusions, and pulmonary metastases, are more characteristic of other thoracic malignancies, such as lung cancer, or other thymic epithelial neoplasms with more aggressive behavior (thymic carcinoma and thymic neuroendocrine tumors). Tomiyama and colleagues demonstrated that the presence of lobulated or irregular contour, areas of low attenuation, and multifocal calcification are suggestive of invasive thymoma. Another study determined that tumor size greater than 7 cm, infiltration of surrounding fat, and lobulated tumor contours were useful in predicting higher-stage thymomas that often are best treated with neoadjuvant therapy. A standardized TNM (tumor, node, metastasis) staging system has recently been developed by the International Association for the Study of Lung Cancer (IASLC) and the ITMIG.

On MRI thymomas typically demonstrate low to intermediate signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. Fat-suppression techniques may be beneficial in distinguishing thymoma from the adjacent mediastinal fat on T2-weighted sequences as the signal intensity of thymoma may approach that of fat. Cystic changes and necrosis result in low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. Intratumoral findings, such as fibrous septations and nodules, are of low signal intensity, whereas hemorrhage demonstrates highly variable signal intensity depending upon age.
Thymic Carcinoma and Thymic Neuroendocrine Neoplasms
Thymic carcinoma is the second most common thymic epithelial neoplasm (after thymoma), representing 20% of such lesions. The most common symptoms described at clinical presentation include those related to compression and invasion of adjacent structures. Neuroendocrine neoplasms of the thymus are the least common type of thymic epithelial neoplasm, most of which are carcinoid tumors. Approximately 25% of these lesions develop in patients with multiple endocrine neoplasia syndrome type 1. Most patients are symptomatic, with reported clinical symptoms at presentation including cough, dyspnea, and chest pain, SVC syndrome, and hoarseness. Affected patients may report symptoms related to paraneoplastic syndromes, the most common of which is Cushing syndrome, characterized by ectopic production of adrenocorticotropic hormone; acromegaly and syndrome of inappropriate antidiuretic hormone are uncommon, and carcinoid syndrome is rare. In contrast to thymoma, thymic carcinoma and thymic neuroendocrine neoplasms tend to demonstrate aggressive features, such as local invasion, intrathoracic lymphadenopathy, and distant metastases.
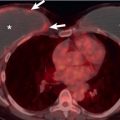
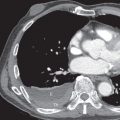
On CT thymic carcinoma manifests as a soft tissue mass that tends to be more heterogeneous than thymoma because of regions of necrosis/cystic change, hemorrhage, and calcification ( Fig. 77.3 ). Thymic neuroendocrine neoplasms may demonstrate invasion of mediastinal fat or vascular structures with a tendency to result in SVC syndrome, and heterogeneous enhancement or hyperenhancement may be present after the administration of IV contrast ( Fig. 77.4 ). The presence of lymphadenopathy and distant metastases suggests an aggressive malignancy, such as thymic carcinoma or thymic neuroendocrine neoplasms; however, pleural metastases are less common than in thymoma.


On MRI thymic carcinoma and thymic neuroendocrine tumors typically appear hyperintense to muscle on both T1- and T2-weighted sequences. Heterogeneous signal intensity secondary to cystic changes, necrosis, and hemorrhage may be present. In contrast to thymic carcinoma, carcinoid tumors may demonstrate hyperenhancement after the administration of IV contrast.
Fat-Containing Masses
Fat-containing masses of the mediastinum demonstrate macroscopic or gross fat measuring between −40 and −120 Hounsfield units (HU) on CT. The most common prevascular masses that manifest with macroscopic intralesional fat include mature teratoma, thymolipoma, lipoma, and liposarcoma.
Mature Teratoma
Mature teratomas are benign neoplasms that usually contain fat, fluid, calcification, and soft tissue on CT, with intralesional fat present in 50% of cases ( Fig. 77.5 ). Fat-fluid levels are highly specific but are much less common. Bone and tooth-like elements may rarely be identified. Mature teratoma accounts for 25% of prevascular masses in patients 10 to 19 years of age. On MRI the prominent fat components are hyperintense on T1-weighted sequences and lose signal on T1-weighted fat-saturated sequences. Proteinaceous fluid and hemorrhage may demonstrate high signal intensity on T1-weighted sequences. Fluid or cystic components demonstrate low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences.

Thymolipoma
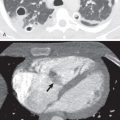
Thymolipoma is a benign encapsulated neoplasm composed of 50% to 85% fat and scattered regions of soft tissue and fibrous septa that is usually encountered in a cardiophrenic angle on CT ( Fig. 77.6 ). These lesions account for less than 5% of prevascular mediastinal masses in all age groups and tend to be very large at presentation, with an average reported size of 20 cm. On MRI the regions of fat are hyperintense on T1-weighted sequences and lose signal on T1-weighted fat-suppressed sequences. The soft tissue components demonstrate intermediate signal intensity of T1- and T2-weighted sequences. A direct connection with the thymus may be visible.

Lipoma and Liposarcoma
Lipomas account for 2% of all primary mediastinal neoplasms and are encapsulated lesions composed predominantly of fat with a small amount of soft tissue and blood vessels on CT. Although liposarcomas are also comprised predominantly of fat, these lesions may be distinguished from lipomas, thymolipomas, and other fat-containing lesions by a greater amount of soft tissue, local invasion, intrathoracic lymphadenopathy, and metastatic disease. On MRI lipoma follows the signal intensity of subcutaneous fat on all sequences. The thin capsule and septations are hypointense on T1- and T2-weighted sequences. In contrast, liposarcoma appears as a more heterogeneous mass, the soft tissue components of which typically enhance after the administration of IV contrast.
Thymic Hyperplasia
Normal thymic tissue is typically present in the prevascular mediastinal compartment in young individuals and decreases with advancing age. In most cases complete fatty replacement is achieved by 40 years of age; however, thymic hyperplasia may result in diffuse thymic enlargement or a focal soft tissue mass in the thymic bed. Two histologic types of thymic hyperplasia have been described: true and thymic lymphoid (or follicular) hyperplasia. True thymic hyperplasia is usually encountered in patients who have been treated with chemotherapy, radiation therapy, or corticosteroids or exposed to stresses such as burns or injuries. This form of thymic hyperplasia is also known as “rebound hyperplasia,” defined as an increase in thymic volume of greater than 50% over baseline after a causative stressor, and affects approximately 10% to 25% of patients undergoing chemotherapy. Follicular hyperplasia is characterized by an increased number of lymphoid follicles, which may or may not be associated with an increase in the size of the gland, usually associated with immunologic diseases, such as myasthenia gravis, hyperthyroidism, collagen vascular diseases, or human immunodeficiency virus (HIV) infection.
True thymic hyperplasia manifests as diffuse symmetric enlargement of the thymus on CT, whereas follicular hyperplasia may result in a normal appearance of the thymus, thymic enlargement, or a focal soft tissue mass in the region of the thymus. Rarely, a heterogeneously hypodense prevascular mediastinal mass may be present on CT because of the deposition of fat between hyperplastic thymic tissue. Thymic hyperplasia may occasionally manifest as a focal soft tissue mass and mimic thymic epithelial neoplasms and other tumors, and chemical shift imaging can be performed to demonstrate the presence of microscopic or intravoxel fat ( Fig. 77.7 ). Chemical shift imaging has been shown to distinguish thymic hyperplasia and normal thymus from other apparent soft tissue masses, as the former demonstrate loss of signal on out-of-phase sequences because of the suppression of microscopic fat interspersed between nonneoplastic thymic tissue, but the latter do not. The chemical shift ratio (CSR) is a tool that can be used to calculate the signal loss and is defined as follows: CSR = (thymus SI OP/paraspinal muscle SI OP)/(thymus SI IP/paraspinal muscle SI IP), in which SI = signal intensity, IP = in-phase sequences, and OP = out-of-phase sequences. The signal loss with thymic hyperplasia and normal thymus results in CSRs of 0.5 to 0.6, whereas thymic epithelial tumors, lymphoma, and other lesions exhibit higher CSRs of 0.9 to 1.0.

Cystic Lesions
Cystic lesions of the mediastinum demonstrate water or fluid attenuation on CT, with HU values between 0 and 20. Prevascular masses that are purely cystic with no soft tissue components or internal septations can reliably be diagnosed as unilocular thymic cysts. When cystic lesions contain soft tissue components or internal septations, the differential diagnosis includes multilocular thymic cyst, cystic teratoma, lymphangioma, and cystic thymoma.
Thymic Cysts
Thymic cysts may be congenital and unilocular or acquired and unilocular or multilocular. Acquired thymic cysts are typically due to inflammation; iatrogenic processes such as surgery, radiation therapy, or chemotherapy; or malignant neoplasms. A well-circumscribed homogeneous lesion in the prevascular mediastinum near the thymic bed that is round, oval, or saccular in appearance on CT likely represents a thymic cyst, although some may demonstrate internal high attenuation resulting from hemorrhagic or proteinaceous components. MRI should be performed to evaluate such lesions because of its ability to distinguish between cystic and solid lesions and identify solid internal components ( Fig. 77.8 ). Thymic cysts typically manifest as well-circumscribed masses in the prevascular mediastinum with low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. Regions of high signal intensity on T1-weighted sequences may be seen in cysts complicated by hemorrhage or infection. The cyst wall is typically visible as a low–signal intensity rim on T2-weighted images. The absence of internal septations and/or soft tissue components is suggestive of unilocular thymic cyst and allows differentiation from other lesions. Minimal enhancement along the peripheral aspect of the cyst may be visualized, although no enhancing internal components should be present. The management of thymic cysts is controversial and highly variable. Cystic lesions demonstrating soft tissue are often evaluated with biopsy and/or surgical resection. In our experience, purely cystic lesions can be reevaluated with follow-up MRI.