Mediastinum and Hila
Jeffrey S. Klein
This chapter reviews the radiologic approach to mediastinal masses, diffuse mediastinal disease, and hilar abnormalities.
Mediastinal Masses
Localized mediastinal abnormalities are common diagnostic challenges for the radiologist. Patients with mediastinal masses tend to present in one of two fashions: with symptoms related to local mass effect or invasion of adjacent mediastinal structures (stridor in a patient with thyroid goiter) or incidentally with an abnormality on a routine chest radiograph. Occasionally, a mediastinal mass is discovered in the course of an evaluation for known malignancy (e.g., a patient with non-Hodgkin lymphoma [NHL]) or for a condition such as myasthenia gravis, in which there is an association with thymoma. Multidetector-row CT (MDCT) is the primary cross-sectional modalities used to evaluate mediastinal masses, with PET useful to assess the response of mediastinal tumors to therapy, particularly lymphoma, and to distinguish residual or recurrent tumor from fibrosis.
For the purposes of the following discussion, the mediastinum is divided into superior (thoracic inlet) and inferior components, with the inferior mediastinum subdivided into anterior, middle, and posterior compartments, as described in Chapter 12 (1).
Thoracic Inlet Masses
The thoracic inlet is the region of the upper thorax marginated by the first rib and represents the junction between the neck and thorax. Masses in this region commonly present as neck masses or with symptoms of upper airway obstruction resulting from tracheal compression. Tortuous/dilated vascular structures, thyroid masses, lymphomatous nodes, and lymphangiomas are the most common thoracic inlet masses (Table 13.1).
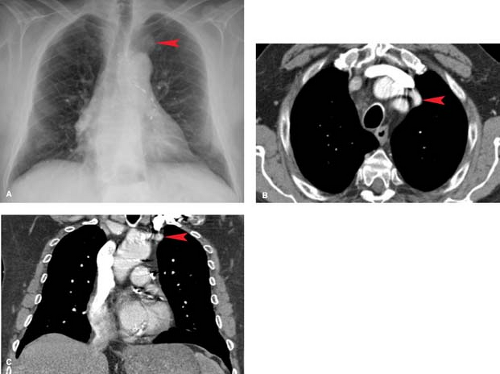
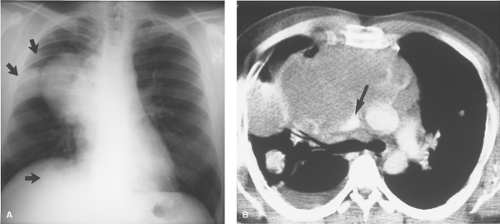
Vascular Structures. Perhaps the most common thoracic inlet mass is seen in older patients as the tortuous arterial structures (Fig. 13.1), in particular the confluence of the right brachiocephalic and right subclavian arteries as they bulge laterally into the right upper lobe to produce a right thoracic inlet mass. Since the “mass” is situated anteriorly in the thoracic inlet, its lateral border above the clavicle is indistinct. This is in distinction to thoracic inlet masses that are posterior or paravertebral in location, which are sharply outlined by apical lung which extends higher posteriorly than anteriorly. This finding is termed the “thoracic inlet” or “cervicothoracic” sign and helps localize thoracic inlet masses, thereby suggesting the etiology of such lesions. Tortuous arterial structures may be identified by the presence of atherosclerotic calcification within their walls and can often be seen on a lateral chest radiograph as a “mass” projecting posterior to the tracheal air column which is sharply outlined posteriorly. In contrast to other masses in the thoracic inlet, a tortuous vessel is usually associated with tracheal deviation toward the side of the mass, whereas most goiters and other inlet masses displace the trachea contralaterally.
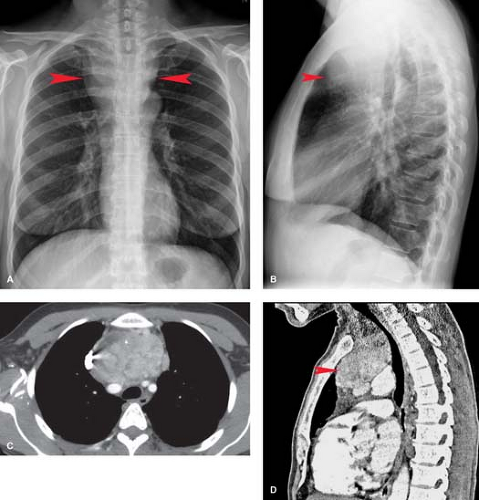
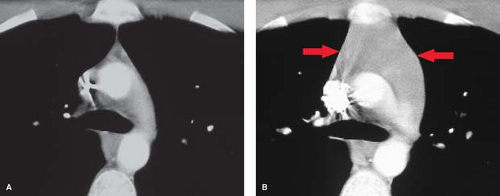
Thyroid Masses. In a small percentage of patients with a cervical thyroid goiter, a thyroid carcinoma, or an enlarged gland from thyroiditis, extension of the thyroid through the thoracic inlet into the superior mediastinum may occur. These lesions are usually discovered as incidental findings on chest radiographs; a minority of patients will present with complaints of dyspnea or dysphagia as a result of tracheal or esophageal compression by the mass. Thyroid goiters arising from the lower pole of the thyroid or the thyroid isthmus can enter the superior mediastinum anterior to the trachea (80% of cases) or to the right and posterolateral to the trachea (20% of cases).
On chest radiographs, an anterosuperior mediastinal mass typically deviates the trachea laterally and either posteriorly (anterior masses) or anteriorly (posterior masses). Coarse, clumped calcifications are common in thyroid goiters. Radioiodine studies should be performed as the initial imaging procedure, although false-negative results do occur. CT usually shows characteristic findings: (1) well-defined margins, (2) continuity of the mass with the cervical thyroid, (3) coarse calcifications, (4) cystic or necrotic areas, (5) baseline high CT attenuation (because of intrinsic iodine content), and (6) intense enhancement (>25 H) as a result of the hypervascularity of most thyroid masses and prolonged enhancement (resulting from active uptake of iodine from contrast media) following IV contrast administration (Fig. 13.2). MR is useful in depicting the longitudinal extension of thyroid goiters without the use of IV contrast.
Parathyroid Masses. In approximately 2% of patients, the parathyroid glands fail to separate from the thymus in the neck and descend with the gland into the anterosuperior mediastinum. These glands can be found near the thoracic inlet in or about the thymus. This becomes important in the small
percentage of patients with persistent clinical and biochemical evidence of hyperparathyroidism following routine neck exploration and parathyroidectomy. Most of these ectopic parathyroid lesions are small (<3 cm) adenomas; rarely, they represent hyperplastic glands or parathyroid carcinoma. When US and nuclear medicine studies have failed to localize a lesion in the neck, CT, MR, or technetium99 sestamibi scanning may be useful in detecting mediastinal lesions.
percentage of patients with persistent clinical and biochemical evidence of hyperparathyroidism following routine neck exploration and parathyroidectomy. Most of these ectopic parathyroid lesions are small (<3 cm) adenomas; rarely, they represent hyperplastic glands or parathyroid carcinoma. When US and nuclear medicine studies have failed to localize a lesion in the neck, CT, MR, or technetium99 sestamibi scanning may be useful in detecting mediastinal lesions.
Table 13.1 Thoracic Inlet Masses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Lymphangiomas. These uncommon masses are tumors comprised of dilated lymphatic channels. The cystic or cavernous form (cystic hygroma) is most commonly discovered in infancy and is often associated with chromosomal abnormalities, including Turner syndrome and trisomies 13, 18, and 21. In infants, these lesions tend to extend from the neck into the anterior mediastinum; less commonly they may arise primarily within the anterior mediastinum in older patients. Histologically, these tumors are composed of cystic spaces lined by epithelium and contain clear, straw-colored fluid. Although these lesions are benign histologically, they tend to insinuate themselves between vascular structures and the trachea. This makes complete surgical resection of lymphangiomas difficult, and they frequently recur. CT demonstrates a well-defined cystic mass within the thoracic inlet or superior mediastinum. MR typically shows a mass of high-signal intensity on T2WIs because of the fluid content.
Anterior Mediastinal Masses
A number of neoplasms and nonneoplastic conditions arise in the anterior mediastinum and produce anterior mediastinal masses. These include thymic neoplasms, lymphoma, germ cell neoplasms, and primary mesenchymal tumors (Table 13.2).
Thymomas or thymic epithelial neoplasms are the second most common primary mediastinal neoplasms in adults after lymphoma. These lesions are neoplasms that arise from the thymic epithelium and contain varying numbers of intermixed lymphocytes. The traditional classification of these tumors is into thymomas, which are histologically benign but may be either encapsulated (noninvasive) or invasive, and thymic carcinomas, in which the epithelial component shows signs of frank malignancy. The World Health Organization has recently reclassified these neoplasms based upon the morphology of the epithelial component and the ratio of epithelial cells to lymphocytes. The classification system divides these neoplasms into types A, AB, B1, B2, B3, and C, with a spectrum of histologic changes ranging from the classic encapsulated thymoma (A), which has a favorable prognosis, to thymic carcinoma (C), which generally carries a poor prognosis (2).
The average age at diagnosis of thymoma is 45 to 50; these lesions are rare in patients under the age of 20. While most often associated with myasthenia gravis, thymoma has been associated with other autoimmune diseases, such as pure red cell aplasia, Graves disease, Sjögren syndrome, and hypogammaglobulinemia. Of patients with myasthenia gravis, 10% to 28% have a thymoma, while a larger percentage of patients with thymoma (30% to 54%) have or will develop myasthenia.
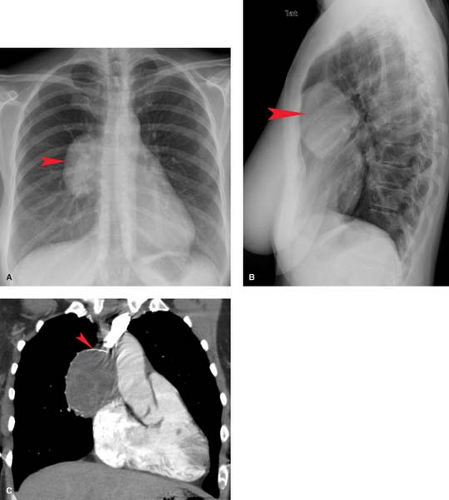
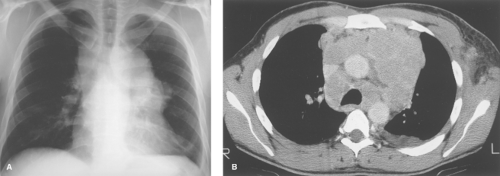
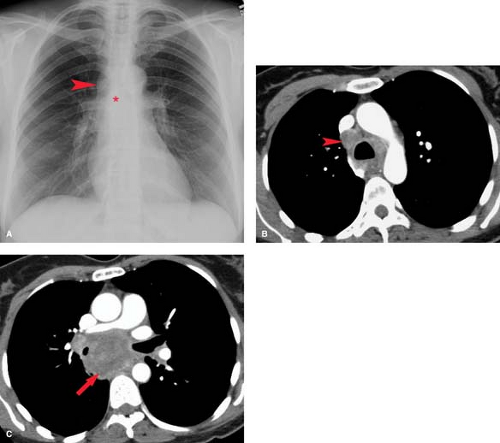
On chest radiographs, thymomas are seen as round or oval, smooth or lobulated soft tissue masses arising near the origin of the great vessels at the base of the heart (2). CT is best for characterizing thymomas and detecting local invasion preoperatively (Fig. 13.3). As a result of their firm consistency, thymomas characteristically maintain their shape where they contact the sternum anteriorly and heart and great vessels posteriorly. Compared to type A tumors, higher-grade thymomas, particularly types B3 and C, tend to show larger size, more irregular margins, heterogeneous enhancement, regions of necrosis, mediastinal nodal metastases, and calcification. Invasion of
tumor through the thymic capsule is present in 33% to 50% of patients (Fig. 13.4; invasive tumor). In the majority of these patients, this determination cannot be made by CT or MR and may even be difficult to determine on examination of the resected specimen. Local invasion of pleura, lung, pericardium, chest wall, diaphragm, and great vessels occurs in decreasing order of frequency in 10% to 15% of patients. Contiguity of a thymoma with the adjacent chest wall or mediastinal structures cannot be used as reliable evidence of invasion of these structures. Drop metastases to dependent portions of the pleural space are a recognized route of spread of thymoma that has invaded the pleura. Extrathoracic metastases are rare, although transdiaphragmatic spread of a pleural tumor into the retroperitoneum has been described. For these reasons, it is important to image the entire thorax and upper abdomen in any patient with suspected invasive disease.
tumor through the thymic capsule is present in 33% to 50% of patients (Fig. 13.4; invasive tumor). In the majority of these patients, this determination cannot be made by CT or MR and may even be difficult to determine on examination of the resected specimen. Local invasion of pleura, lung, pericardium, chest wall, diaphragm, and great vessels occurs in decreasing order of frequency in 10% to 15% of patients. Contiguity of a thymoma with the adjacent chest wall or mediastinal structures cannot be used as reliable evidence of invasion of these structures. Drop metastases to dependent portions of the pleural space are a recognized route of spread of thymoma that has invaded the pleura. Extrathoracic metastases are rare, although transdiaphragmatic spread of a pleural tumor into the retroperitoneum has been described. For these reasons, it is important to image the entire thorax and upper abdomen in any patient with suspected invasive disease.
Table 13.2 Anterior Mediastinal Masses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In patients with myasthenia gravis who are being evaluated for thymoma, CT can demonstrate tumors that are invisible on conventional radiographs. However, very small thymic tumors may not be distinguishable from a normal or hyperplastic gland with CT, particularly in younger patients with a large amount of residual thymic tissue.
Thymic cysts may be congenital or acquired. Congenital unilocular thymic cysts are rare lesions that represent remnants of the thymopharyngeal duct and contain thin or gelatinous fluid. They are characterized histologically by an epithelial lining, with thymic tissue in the cyst wall, which distinguishes thymic cysts histologically from other congenital cystic lesions within the anterior mediastinum. Acquired multilocular thymic cysts are postinflammatory in nature and have been associated with AIDS, prior radiation or surgery, and autoimmune conditions such as Sjögren syndrome, myasthenia gravis, and aplastic anemia. In these latter conditions, clinical and radiologic distinction of multilocular thymic cyst from thymoma may be difficult; in fact, the two conditions can coexist. Large cysts will be evident as soft tissue masses on conventional radiographs, and CT or MR will demonstrate the cystic nature of the lesion (3). If the distinction between a true thymic cyst, cystic degeneration of a thymoma or lymphoma, a germ cell neoplasm, or lymphangioma is impossible on clinical and radiologic grounds, the lesion should be biopsied or resected.
Thymolipoma is a rare, benign thymic neoplasm that consists primarily of fat with intermixed rests of normal thymic tissue. These masses are asymptomatic and therefore are typically large when first detected. Chest radiographs show a large anterior mediastinal mass that, because of its pliable nature, tends to envelope the heart and diaphragm. CT demonstrates a fatty mass with interspersed soft tissue densities.
Resection is curative.
Thymic Carcinoid. Neuroendocrine tumors of the thymus are rare malignant neoplasms believed to arise from thymic cells of neural crest origin (amine precursor uptake and decarboxylation—APUD or Kulchitsky cells). The most common histologic type is carcinoid tumor, which, as with similar lesions arising within the bronchi, ranges in differentiation and behavior from typical carcinoid to atypical carcinoid to small cell carcinoma. Approximately 40% of patients have Cushing syndrome as a result of adrenocorticotropic hormone secretion by the tumor; these patients tend to have smaller lesions at the time of diagnosis since they present early with signs of corticosteroid excess. The carcinoid syndrome is uncommon. This lesion is indistinguishable from thymoma on plain radiographs and CT scans.
Thymic hyperplasia is defined as enlargement of a thymus that is normal on gross and histologic examination. This rare entity occurs primarily in children as a rebound effect in response to an antecedent stress, discontinuation of chemotherapy, or treatment of hypercortisolism. An association with Graves disease has also been noted. The term thymic hyperplasia has been used incorrectly to describe the histologic findings of lymphoid follicular hyperplasia of the thymus, found in 60% of patients with myasthenia gravis. In contrast to most cases of true thymic hyperplasia, lymphoid hyperplasia does not produce thymic enlargement. Most patients with thymic hyperplasia have normal or diffusely enlarged glands on CT (Fig. 13.5); occasionally thymic hyperplasia will present as a mass that is radiographically indistinguishable from thymoma. Most cases can be resolved by noting a decrease in size on follow-up studies, thereby obviating the need for biopsy.
Thymic Lymphoma. The thymus is involved in 40% to 50% of patients with the nodular sclerosing subtype of Hodgkin disease. Its radiographic appearance is indistinguishable from that of other solid neoplasms arising within the thymus. The presence of lymph node enlargement in other portions of the mediastinum or anterior chest wall involvement should suggest the diagnosis.
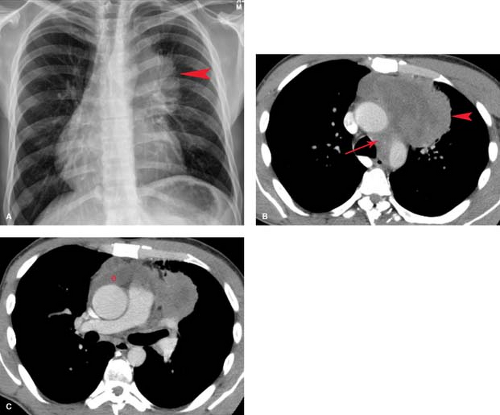
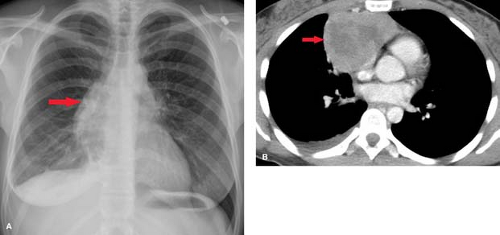
Lymphoma—either Hodgkin disease or non-Hodgkin lymphoma (NHL)—is the most common primary mediastinal neoplasm in adults. Hodgkin disease involves the thorax in 85% of patients at the time of presentation. The majority (90%) of patients with intrathoracic involvement have mediastinal lymph node enlargement; this most commonly involves the anterior mediastinal and hilar nodal groups. The anterior mediastinum is the most frequent site of a localized nodal mass in patients with Hodgkin disease, particularly those with the nodular sclerosing type (Fig. 13.6). Isolated enlargement of mediastinal or hilar nodes outside the anterior mediastinum should suggest an alternative diagnosis. Only 25% of patients with Hodgkin lymphoma have disease limited to the mediastinum at the time of diagnosis. NHL involves the thorax in approximately 40% of patients at presentation. In contrast to
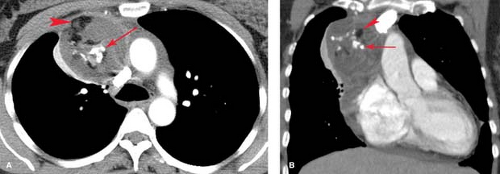
Hodgkin disease, only 50% of patients with NHL and intrathoracic disease have mediastinal nodal involvement, and only 10% of NHL patients have disease that is limited to the mediastinum. Of the various subtypes of NHL that present with mediastinal masses, lymphoblastic lymphoma and diffuse large B-cell lymphoma are the most common (Fig. 13.7). Lymphoma involving a single mediastinal or hilar nodal group is much more common in NHL than in Hodgkin disease. NHL most commonly involves middle mediastinal and hilar lymph nodes; juxtaphrenic and posterior mediastinal nodal involvement is uncommon but is seen almost exclusively in NHL. Patterns of pulmonary parenchymal involvement in lymphoma are discussed in Chapter 15.
Hodgkin disease, only 50% of patients with NHL and intrathoracic disease have mediastinal nodal involvement, and only 10% of NHL patients have disease that is limited to the mediastinum. Of the various subtypes of NHL that present with mediastinal masses, lymphoblastic lymphoma and diffuse large B-cell lymphoma are the most common (Fig. 13.7). Lymphoma involving a single mediastinal or hilar nodal group is much more common in NHL than in Hodgkin disease. NHL most commonly involves middle mediastinal and hilar lymph nodes; juxtaphrenic and posterior mediastinal nodal involvement is uncommon but is seen almost exclusively in NHL. Patterns of pulmonary parenchymal involvement in lymphoma are discussed in Chapter 15.
While Hodgkin disease spreads in a fairly predictable pattern from one nodal group to an adjacent group, NHL is felt to be a multifocal disorder in which patterns of involvement are unpredictable. Localized intrathoracic Hodgkin disease is usually treated with radiation therapy, with 90% response rates. More widespread Hodgkin disease and NHL are treated with chemotherapy, with better response rates for Hodgkin disease than for NHL.
On conventional radiographs, lymphoma involving the anterior mediastinum is indistinguishable from thymoma or germ cell neoplasm and presents as a lobulated mass projecting to one or both sides (Figs. 13.6, 13.7). Calcification in untreated lymphoma is extremely uncommon, and its presence within an anterior mediastinal mass should suggest another diagnosis. Involvement of other lymph nodes in the mediastinum or hila makes lymphoma more likely. An enlarged spleen displacing the gastric air bubble medially, seen in the upper abdominal portion of the frontal chest film, provides an additional clue to the diagnosis.
CT is performed in virtually all patients with lymphoma. The advantages of chest CT include the ability to better characterize and localize masses seen on chest radiographs; detection of subradiographic sites of involvement that can alter disease
staging, prognosis, and therapy; guidance for transthoracic or open biopsy; the ability to monitor response to therapy; and detection of relapse. The appearance of nodal involvement in lymphoma varies; most commonly, discrete enlarged solid lymph nodes or conglomerate masses of nodes are seen (Fig. 13.6B). Central necrosis, seen in 20% of patients, has no prognostic significance. Nodal calcification is rare in the absence of previous mediastinal radiation or systemic chemotherapy. Parenchymal involvement is usually the result of direct extranodal extension of a tumor from hilar nodes along the bronchovascular lymphatics; this is better appreciated on axial CT images than on chest radiographs (4,5). Likewise, a tumor extending from the mediastinum to the pericardium, subpleural space, and chest wall is best appreciated on CT or MR. On MR, untreated lymphoma appears as a mass of uniform low-signal intensity on T1WIs and uniform high-signal intensity or intermixed areas of low- and high-signal intensity on T2WIs. The areas of low-signal intensity on T2WIs of untreated patients may be a result of foci of fibrotic tissue in nodular sclerosing Hodgkin disease.
staging, prognosis, and therapy; guidance for transthoracic or open biopsy; the ability to monitor response to therapy; and detection of relapse. The appearance of nodal involvement in lymphoma varies; most commonly, discrete enlarged solid lymph nodes or conglomerate masses of nodes are seen (Fig. 13.6B). Central necrosis, seen in 20% of patients, has no prognostic significance. Nodal calcification is rare in the absence of previous mediastinal radiation or systemic chemotherapy. Parenchymal involvement is usually the result of direct extranodal extension of a tumor from hilar nodes along the bronchovascular lymphatics; this is better appreciated on axial CT images than on chest radiographs (4,5). Likewise, a tumor extending from the mediastinum to the pericardium, subpleural space, and chest wall is best appreciated on CT or MR. On MR, untreated lymphoma appears as a mass of uniform low-signal intensity on T1WIs and uniform high-signal intensity or intermixed areas of low- and high-signal intensity on T2WIs. The areas of low-signal intensity on T2WIs of untreated patients may be a result of foci of fibrotic tissue in nodular sclerosing Hodgkin disease.
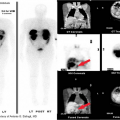
CT and fluorodeoxyglucose (FDG) PET are used to monitor the response of lymphoma to therapy. While CT can accurately assess tumor regression and detect relapse within nodal groups outside the treated region, the ability to distinguish residual tumor from sterilized fibrotic masses is limited. Residual soft tissue masses have been reported in up to 50% of patients, most commonly with nodular sclerosing Hodgkin disease, and are more common when the pretreatment mass is large. Some patients with residual masses on CT or MR will have tumor recurrence within 6 to 12 months after the completion of therapy. In general, the appearance of high-signal intensity regions on T2WIs more than 6 months after treatment should suggest recurrence. Radionuclide scintigraphy with gallium-67, particularly SPECT, has been largely replaced by FDG-PET in the initial diagnosis and staging of thoracic lymphoma. PET is clearly superior to CT or MR in distinguishing recurrent tumor from fibrosis in both Hodgkin disease and NHL.
Germ cell neoplasms, which include teratoma, seminoma, choriocarcinoma, endodermal sinus tumor, and embryonal cell carcinoma, arise from collections of primitive germ cells
that arrest in the anterior mediastinum on their journey to the gonads during embryologic development. Since they are histologically indistinguishable from germ cell tumors arising in the testes and ovaries, the diagnosis of a primary malignant mediastinal germ cell neoplasm requires exclusion of a primary gonadal tumor as a source of mediastinal metastases. A key in distinguishing primary from metastatic mediastinal germ cell neoplasm is the presence of retroperitoneal lymph node involvement in metastatic gonadal tumors.
that arrest in the anterior mediastinum on their journey to the gonads during embryologic development. Since they are histologically indistinguishable from germ cell tumors arising in the testes and ovaries, the diagnosis of a primary malignant mediastinal germ cell neoplasm requires exclusion of a primary gonadal tumor as a source of mediastinal metastases. A key in distinguishing primary from metastatic mediastinal germ cell neoplasm is the presence of retroperitoneal lymph node involvement in metastatic gonadal tumors.
The most common benign mediastinal germ cell neoplasm is teratoma, comprising 60% to 70% of mediastinal germ cell neoplasms. Teratomas may be cystic or solid. Cystic or mature teratoma is the most common type of teratoma seen in the mediastinum. In contrast to a dermoid cyst, which is an ovarian neoplasm containing only elements derived from the ectodermal germinal layer, a cystic teratoma of the mediastinum commonly contains tissues of ectodermal, mesodermal, and endodermal origins. For this reason, it is inaccurate to use the term “dermoid cyst” to describe cystic mediastinal germ cell neoplasms. Solid teratomas are usually malignant, with seminoma comprising 25% to 50% of such lesions (6). Most germ cell neoplasms are detected in patients in the third or fourth decade of life. While benign tumors have a slight female preponderance (female/male, 60%/40%), malignant tumors are seen almost exclusively in men.
Radiographically, these tumors have a distribution similar to that of thymomas. While the majority are located in the anterior mediastinum, up to 10% are found in the posterior mediastinum. Benign lesions are often round or oval and smooth in contour; an irregular, lobulated, or ill-defined margin suggests malignancy. Calcification is present in 33% to 50% of tumors but is nonspecific unless in the form of a tooth. On CT, benign teratomas are usually cystic and may contain soft tissue, bone, teeth, fat, or, rarely, fat–fluid levels (Fig. 13.8). Seminoma, choriocarcinoma, and endodermal sinus (yolk sac) tumors are malignant lesions seen primarily in young men. Seminoma is the most common malignant germ cell neoplasm, accounting for 30% of these tumors. The radiographic findings are nonspecific. CT typically shows a large, lobulated soft tissue mass that may contain areas of hemorrhage, calcification, or necrosis (Fig. 13.9). Elevated serum levels of α-fetoprotein or human chorionic gonadotropin are helpful in the diagnosis of suspected malignant mediastinal germ cell neoplasm, while clinical and CT evidence of gynecomastia is an additional clue.
Thyroid Masses. While masses arising from the thyroid can present as anterior and superior mediastinal masses, these lesions are best considered as thoracic inlet masses, as discussed earlier.
Mesenchymal Tumors. Benign and malignant tumors arising from the fibrous, fatty, muscular, or vascular tissues of the mediastinum may present as mediastinal masses, most commonly in the anterior mediastinum. Lipomas can occur in any location in the mediastinum but are most often anterior. The diagnosis is made by the recognition of a well-defined mass of uniform fatty attenuation (under -50 H). The presence of soft tissue elements should raise the possibility of a thymolipoma or liposarcoma; the latter may show evidence of invasion of adjacent structures at the time of diagnosis. Fat within a mature teratoma or transdiaphragmatic herniation of omental fat is usually easily distinguished from a lipoma.
Hemangiomas are benign tumors composed of vascular channels and may be associated with the syndrome of hereditary hemorrhagic telangiectasia. A pathognomonic sign on chest radiographs is the recognition of phleboliths within a smooth or lobulated soft tissue mass. Angiosarcomas are rare malignant vascular neoplasms that are indistinguishable from other invasive neoplasms arising within the anterior mediastinum.
Leiomyomas are rare benign neoplasms that arise from smooth muscle within the mediastinum. Similarly, fibromas and mesenchymomas (tumors that contain more than one mesenchymal element) can appear as anterior mediastinal masses.
Middle Mediastinal Masses
Lymph Node Enlargement and Masses (Table 13.3). Most middle mediastinal lymph node masses are malignant, representing metastases from bronchogenic carcinoma (Fig. 13.10), extrathoracic malignancy, or lymphoma (7). Benign causes of middle mediastinal lymph node enlargement include sarcoidosis, mycobacterial and fungal infection, angiofollicular lymph node hyperplasia (Castleman disease), and angioimmunoblastic lymphadenopathy.
On plain radiographs, several findings suggest that a middle mediastinal mass represents lymph node enlargement. The presence of multiple bilateral mediastinal masses that distort the lung/mediastinal interface is relatively specific for lymph node enlargement. Solitary masses resulting from lymph node enlargement tend to be elongated and lobulated rather than spherical, since usually more than a single node in a vertical chain of nodes is involved. Occasionally, calcification can be detected within enlarged lymph nodes on plain radiographs;
CT is more sensitive in detecting nodal calcification and its distribution within lymph nodes.
CT is more sensitive in detecting nodal calcification and its distribution within lymph nodes.
Table 13.3 Middle Mediastinal Masses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
One of the prime indications for performing thoracic CT is to detect the presence of enlarged mediastinal lymph nodes. CT is most often obtained to confirm an abnormal chest radiographic finding or to evaluate a patient with suspected mediastinal disease despite normal radiographs (a patient with a suspicious solitary pulmonary nodule or with cervical Hodgkin disease). The ability of CT to image in the axial plane and its inherent high-contrast resolution allow for the recognition of abnormally enlarged lymph nodes that would not be evident on chest radiographs. In general, abnormal lymph nodes are seen as round or oval soft tissue masses that measure larger than 1.0 cm in their short-axis diameter. Although CT is unable to distinguish between benign inflammatory nodes and those involved by malignancy based upon size criteria alone, CT can provide useful information about the internal density of the nodes (Table 13.4).
Table 13.4 Density of Mediastinal/Hilar Nodes on Ct | ||||||||
|---|---|---|---|---|---|---|---|---|
|
A standardized classification system for hilar and mediastinal lymph nodes is the American Thoracic Society (ATS) map (Fig. 13.11). This scheme correlates with easily identifiable CT and anatomic landmarks and is most important when reporting lymph node enlargement in patients with bronchogenic carcinoma. A recently recommended new lymph node map has been proposed by the International Association for the Staging of Lung Cancer (8). A diagram of this simplified seven-station nodal map is illustrated in Chapter 15, Figure 15.20.
MR is as sensitive as CT in detecting enlarged mediastinal lymph nodes. Advantages of MR include the absence of iodinated contrast, easy distinction between vascular and soft tissue structures, exquisite contrast resolution between mediastinal nodes and fat on T1W sequences, and the ability to image in the direct coronal or sagittal plane. The latter feature is an advantage in those mediastinal regions that parallel the axial plane (subcarinal space, aortopulmonary window) and therefore tend to suffer from partial volume-averaging effects on CT. The major disadvantages of MR at present are the inability to detect nodal calcification and limited spatial resolution; the latter can result in an inability to distinguish between a group of normal size nodes and a single enlarged node, thereby leading to false-positive results.
In addition to the detection and characterization of enlarged mediastinal nodes, CT can help guide diagnostic nodal tissue sampling. This is usually most helpful in the setting of suspected bronchogenic carcinoma, where accurate staging of mediastinal nodal disease is important for prognostic purposes and treatment planning. The recognition of enlarged subcarinal or pretracheal nodes on CT may suggest biopsy via transcarinal Wang needle or mediastinoscopy, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree