Chapter Outline
Malignant Peritoneal Mesothelioma
Primary Peritoneal Serous Carcinoma
Well-Differentiated Papillary Mesothelioma
Multicystic Peritoneal Mesothelioma
Desmoplastic Small Round Cell Tumor
Inflammatory and Infiltrative Diseases
Anatomy
The mesentery is a part of the peritoneal lining that extends from the posterior peritoneum and suspends bowel loops. The mesentery is composed of two thin layers of fibrofatty tissue, which surrounds and contains the vascular and lymphatic structures supplying either the small bowel or colon. The purpose of the peritoneum and mesentery is to provide a smooth and frictionless surface between the solid organs. The mesentery can be further divided according to whether it suspends small intestine or colon. The small bowel mesentery is obliquely placed in the peritoneal cavity and extends from the ligament of Treitz in the left upper quadrant to the terminal ileum and ileocecal valve in the right lower quadrant. The transverse mesocolon extends from the pancreas and suspends the transverse mesocolon. The root of the transverse mesocolon passes across the second portion of the duodenum and the head, body, and tail of the pancreas. The plane of the transverse mesocolon can be identified by following the middle colic vessels.
The greater omentum is the continuation of the dorsal mesogastrium inferiorly from the greater curvature of the stomach. This extends inferiorly and doubles back anterior to the transverse colon and then continues posteriorly as the transverse mesocolon. The descending and ascending portions of the greater omentum usually fuse to form a four-layer vascular peritoneal fold ( Fig. 111-1 ).
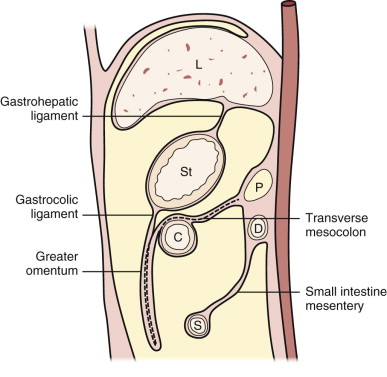
The peritoneal cavity is divided primarily by the transverse mesocolon into the supramesocolic and inframesocolic compartments. The peritoneal folds in the supramesocolic compartment consist of the falciform, coronary, gastrohepatic, hepatoduodenal, greater omentum, gastrosplenic, splenorenal, and phrenicocolic ligaments. The falciform ligament divides the subphrenic space into the right and left subphrenic spaces. The phrenicocolic ligament extends from the splenic flexure to the diaphragm and limits the cranial extent of the left paracolic gutter. The inframesocolic compartment is subdivided by the small bowel mesentery into the right and the larger left portion extending deep into the pelvis. The left inframesocolic space is limited in the lower left pelvis by the sigmoid mesocolon.
All the ligaments of the peritoneal cavity consist of fused peritoneal layers and form the subperitoneal space, which is a continuous potential space that connects the intraperitoneal compartment to the retroperitoneal compartment. The subperitoneal space also extends to the solid viscera. Thus, any disease process that involves the subperitoneal space can spread bidirectionally to involve either the peritoneal compartment or the retroperitoneum and may involve the abdominal organs.
In most patients, there is sufficient macroscopic fat on computed tomography (CT) to identify the small bowel mesentery, transverse mesocolon, and sigmoid mesocolon. Mesenteric fat has Hounsfield unit measurements of fat elsewhere and measures between −100 and −160 HU.
Primary Neoplasms
Primary tumors arising from the peritoneum are rare and are usually of mesenchymal origin.
Desmoid Tumors
These tumors are related to benign but locally aggressive fibroblast proliferation or fibromatosis. The morbidity associated with these tumors is related to their locally aggressive behavior with involvement of adjacent organs. They are uncommon, occurring in 2 to 4 per million people per year, and do not show the features of neoplasia. The cause is unknown, but there is an association with pregnancy and estrogen administration. They can occur either sporadically or in association with familial adenomatous polyposis. Familial adenomatous polyposis is an inherited syndrome characterized by innumerable polyps predominantly occurring in the colon. Gardner’s syndrome is now considered to be a part of familial adenomatous polyposis, and both these conditions have a mutation of the APC gene.
Desmoid tumors can occur in 5% to 25% of patients with familial adenomatous polyposis. About half of the abdominal desmoids occur intra-abdominally, and the other half are found in the abdominal wall. Nearly one third of abdominal desmoids cause pain. Abdominal desmoids can involve the abdominal wall, mesentery, or retroperitoneum ( Figs. 111-2 and 111-3 ). Many of these tumors are often associated with prior surgery, such as colectomy, and can recur at the surgical site. Surgery is thought to stimulate desmoid tumor growth. The most common site for mesenteric desmoids is at the base of the small bowel mesentery. Tumors can vary in size and range from a few centimeters to extremely large lesions. Desmoids are pseudoencapsulated lesions despite their relatively well defined gross appearance. On microscopic examination, they demonstrate infiltrative margins. Progressive growth may lead to bowel, ureteric, or vascular obstruction and occasionally fistulas. CT is an excellent imaging study to evaluate desmoid tumors and their relation to surrounding structures and in the follow-up of patients who undergo conservative medical therapy. Desmoid tumors are typically poorly enhancing solid lesions. They can usually be distinguished from postoperative fibrosis by the lack of mass effect of the fibrosis. On ultrasound, these appear as well-demarcated solid masses ( Fig. 111-4 ) containing low to mid-level echoes. On magnetic resonance imaging (MRI), they are of low T1 and low T2 signal intensity ( Fig. 111-5 ) with no or poor enhancement.




Malignant Peritoneal Mesothelioma
Malignant peritoneal mesothelioma (MPM) is a rare but aggressive tumor that arises from the serosa lining the pleura and the peritoneum. MPMs account for only 10% to 15% of all mesotheliomas. These may occur either alone or in conjunction with pleural mesothelioma. Most patients have a history of exposure to asbestos. There is also a long latent period between exposure and appearance of peritoneal mesothelioma, typically 30 years after the exposure to asbestos. There is a slight male predominance, less than that of pleural mesotheliomas. It has been postulated that the patients with MPM have to have a higher cumulative exposure to asbestos than that of the patients with pleural mesothelioma.
Peritoneal mesotheliomas can be classified into diffuse versus localized mesothelioma. The diffuse type is aggressive as opposed to the localized form, which has a better prognosis with surgery.
Peritoneal mesotheliomas can be classified into epithelial, sarcomatoid, and mixed types on the basis of histologic features. There is great variability in the histologic appearance, making the diagnosis difficult. Sarcomatoid histology is associated with a poor prognosis. Immunohistochemical staining has been proved to be of great benefit in differentiating MPMs from secondary tumors affecting the peritoneum. MPMs produce large amounts of hyaluronic acid and stain with colloidal iron. In contrast, MPMs do not stain positive for the presence of mucin. Symptoms are nonspecific and include abdominal distention, pain, and malaise. Fifty-five percent of patients fail to demonstrate evidence of asbestosis on chest radiographs.
CT is the most useful test in the initial evaluation of patients with increasing abdominal girth and pain. The nodules and masses in mesothelioma enhance with intravenous administration of contrast material. These can be manifested as sites of peritoneal thickening, infiltration, or nodularity ( Fig. 111-6 ). A sheetlike pattern of growth with thickened appearance to the mesentery and bowel wall can also be seen. Bowel loops may be fixed from these infiltrative changes. Calcification is rare in MPMs. They can be associated with variable amounts of ascites. Scalloping or mass effect on adjacent organs is typically seen. On MRI, the nodules are of low T1 signal intensity and intermediate to high T2 signal intensity ( Fig. 111-7 ). The peritoneal nodules can also be seen on dynamic or diffusion-weighted imaging. Positron emission tomography (PET) has a limited role in exact anatomic evaluation of MPMs. All the imaging modalities are limited in their detection of peritoneal nodules less than 0.5 cm. Peritoneal mesothelioma is associated with poor survival; median survival time is 8 to 12 months after diagnosis.


CT is of help in evaluating for a primary tumor elsewhere in the gastrointestinal or genitourinary tracts, breast, and pancreas. The differential diagnosis includes metastatic disease, primary papillary serous carcinoma of the peritoneum, lymphoma, and granulomatous disease. The presence of marked and enlarged lymphadenopathy favors lymphoma or metastatic disease as the most likely diagnosis. Similarly, calcification of the peritoneal thickening makes MPMs less likely.
The mesenteric infiltration results in a characteristic stellate and fixed appearance.
Primary Peritoneal Serous Carcinoma
Primary peritoneal serous carcinoma is a rare malignant neoplasm that typically occurs in postmenopausal women. This is a primary tumor of the peritoneum, but the cell origin is thought to be the extraovarian mesothelium with müllerian potential.
According to the Gynecologic Oncology group, for a diagnosis of primary peritoneal serous carcinoma to be made, the ovaries should be normal in size or enlarged from a benign process, and the involvement of the extraovarian sites should be greater than ovarian involvement. The prognosis and treatment are similar to those of serous ovarian carcinoma. Calcification can be seen with this tumor and can help differentiate MPM from this tumor ( Fig. 111-8 ). The CT and the pathologic appearance of primary peritoneal serous carcinoma is similar to that of carcinomatosis from serous ovarian cancer. Assessment for primary ovarian masses should be performed to exclude peritoneal carcinomatosis from ovarian cancer. Psammomatous calcifications can be seen in up to 30% of cases.

Well-Differentiated Papillary Mesothelioma
This is a rare subtype of mesothelioma that is clinically distinct from MPM. These tumors are seen typically in younger women and are not associated with asbestos exposure. They may be detected and removed incidentally. These tumors tend to be small ( Fig. 111-9 ). There is very little written about these tumors in the radiologic literature. These tumors can be manifested as calcifications of the peritoneal surface without associated mass but can also be multiple peritoneal nodules. The clinical course tends to be indolent after surgery. However, these tumors should be observed because of the small risk for development into MPMs.

Multicystic Peritoneal Mesothelioma
This is another rare subtype of peritoneal mesothelioma occurring predominantly in young and middle-aged women along the pelvic peritoneal surface. There is no association to asbestos exposure in these patients. A higher incidence of prior abdominal surgery or pelvic inflammatory disease is seen in these patients. This tumor is composed of cysts that vary in size and are separated by septations or fibrous tissue. It can appear as a multicystic mass, multiple unilocular cysts adjacent to each other, or a unilocular cystic mass. On ultrasound, this tumor appears as a multiloculated lesion containing anechoic cystic spaces separated by echogenic septations. They can surround the ovaries, and the ovaries may appear entrapped within the cystic mass. On CT, this appears as a well-defined, noncalcified multiloculated cystic mass with enhancing internal septations. Fifty percent of these tumors tend to locally recur after surgical resection.
There is a slight risk for transformation into a malignant mesothelioma, and long-term follow-up should be performed in these patients.
Desmoplastic Small Round Cell Tumor
This is a rare but extremely aggressive tumor that typically occurs in adolescents and young adults and more commonly in men. The most common clinical complaint is abdominal distention. The prognosis is poor, and the 3-year survival is less than 30%. CT shows multiple large solid intraperitoneal masses ( Fig. 111-10 ) without an apparent primary site. The tumor predominantly involves the omentum and paravesical space. Areas of central low attenuation may correspond to hemorrhage or necrosis. Ascites and hepatic metastases are associated findings. There is both hematogenous and intraperitoneal spread that can be seen with this tumor. Retroperitoneal and paratesticular involvement has been reported with this tumor. Malignant ascites is frequently seen with these tumors. They tend to be of low T1 and high T2 signal intensity and demonstrate heterogeneous contrast enhancement.

Adenomatoid Tumor
This is a very rare tumor that is typically discovered incidentally. Adenomatoid tumor can occur in conjunction with the multicystic mesotheliomas. It is typically small and characterized by epithelioid cells.
Other Primary Mesenchymal Tumors
Tumors may arise from the mesenchymal structures and may be of fatty, vascular, lymphatic, or neurogenic tissue origin.
Tumors of fatty origin can range from benign lipoma to the malignant liposarcoma. Benign lipoma is usually a well-defined homogeneous lesion containing tissue of fat attenuation on CT. Liposarcomas can be ill-defined heterogeneous lesions with a variable soft tissue component ( Fig. 111-11 ). The diagnosis of liposarcoma can be made on CT if this mass contains fat interspersed with the soft tissue component or areas of enhancement.

Hemangiomas can occur in the mesentery. These are further divided into cavernous, capillary, and venous types. Cavernous hemangiomas containing large vascular sinusoids are the most common type of hemangioma to occur in the mesentery. They are typically of soft tissue attenuation and may contain phleboliths within them. Hemangiopericytomas can also occur in the mesentery and are typically large, vascular masses ( Fig. 111-12 ).

Lymphangiomas are benign tumors of lymphatic origin. These can be either congenital tumors or benign tumors of mesenchymal origin. They are typically multiloculated cystic masses with thin enhancing walls ( Fig. 111-13 ). It can be difficult to differentiate them from cystic mesotheliomas.

Tumors of neurogenic origin are typically benign and are more common in the retroperitoneum than in the mesentery. On CT, these tumors are solid low-attenuation masses ( Fig. 111-14 ) that show minimal contrast enhancement. They are of low T1 signal intensity and intermediate to high T2 signal intensity and show minimal contrast enhancement. They may show a tubular or elongated pattern. Uncommonly, they may show malignant degeneration.

Leiomyomatosis peritonealis disseminata is a rare entity characterized by multiple smooth muscle tumor nodules throughout the peritoneum. This is typically found in young women with uterine fibroids and detected incidentally.
Sarcomas are more common in the retroperitoneum compared with the mesentery. The tumors often arise in the retroperitoneum and extend into the peritoneum. They are typically large at the time of diagnosis ( Fig. 111-15 ) and can show areas of central low attenuation related to necrosis or hemorrhage. Malignant fibrous histiocytoma is the most common peritoneal sarcoma.