Metabolic Bone Disease
Clyde A. Helms
Osteoporosis
Osteoporosis is defined as diminished bone quantity in which the bone is otherwise normal. This contrasts to osteomalacia in which the bone quantity is normal but the quality of the bone is abnormal in that it is not normally mineralized. Osteomalacia results in excess nonmineralized osteoid. It is not possible in most cases to distinguish between osteoporosis and osteomalacia on plain films; hence, many prefer the term “osteopenia” for the plain film finding of diminished mineralization.
There are myriad causes of osteoporosis, the most common of which is primary osteoporosis (the so-called senile osteoporosis or osteoporosis of aging). This is seen most commonly in postmenopausal women and is a major health concern because of the increase of vertebral body and hip fractures in this patient population.
Secondary osteoporosis implies that an underlying disorder, such as thyrotoxicosis or renal disease, has caused the osteoporosis. Only about 5% of the cases of osteoporosis are of the secondary type. The differential diagnosis for secondary osteoporosis is quite long and probably should not be memorized. One cannot even be sure whether it is osteoporosis or osteomalacia on the basis of the plain films; therefore, the differential for presumed osteoporosis would have to include the causes of osteomalacia.
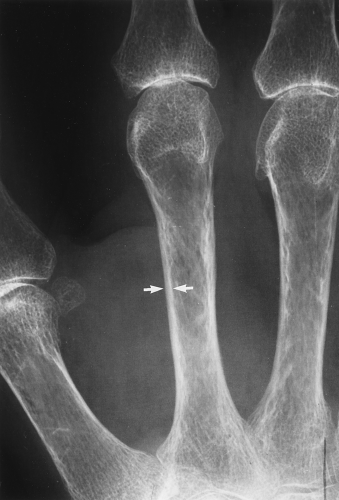
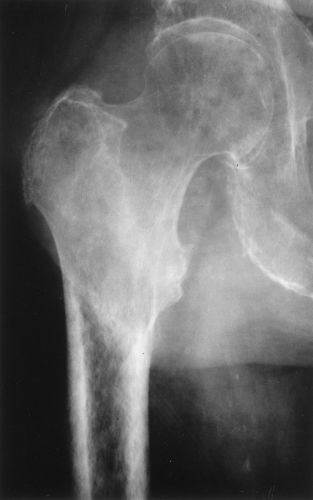
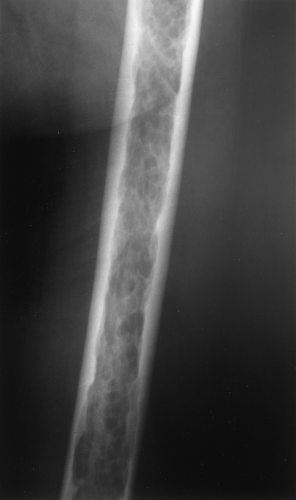
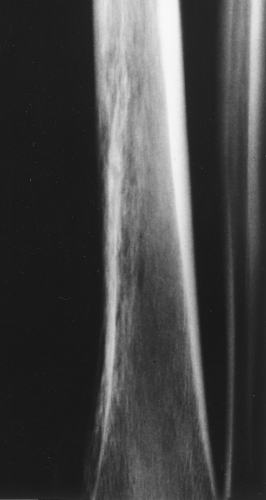
The main radiographic finding in osteoporosis is thinning of the cortex. Although this can be seen in any bone, it is most reliably demonstrated in the second metacarpal at the middiaphysis. The normal metacarpal cortical thickening should be approximately one-fourth to one-third the thickness of the metacarpal (Fig. 44.1). In osteoporosis, this cortical thickness is decreased (Fig. 44.2). The metacarpal cortex (and all bony cortices, for that matter) decreases in thickness normally with age and is thinner in females than in males of the same age. Several tables have been published that give the normal metacarpal cortical measurement that have age and sex adjustments to allow the determination of normal. Unfortunately, these only determine the mineralization of the peripheral skeleton and do not seem to relate to whether vertebral body or hip fractures will occur.
Measurement of the bone mineral content in the axial skeleton can be done by one of several methods that use CT to assess the bone quantity in the spine. There is much debate about which method is superior and even about whether knowing the bone mineral content is clinically more helpful than just knowing the age and sex of the patient, which is fairly accurate for predicting the bone-mass quantity.
Exercise and proper diet seem to help delay the onset of primary osteoporosis. Calcium additives have not been shown to reverse the process of primary osteoporosis. Estrogen clearly plays a role in alleviating postmenopausal osteoporosis, yet its use in a widespread manner is somewhat controversial.
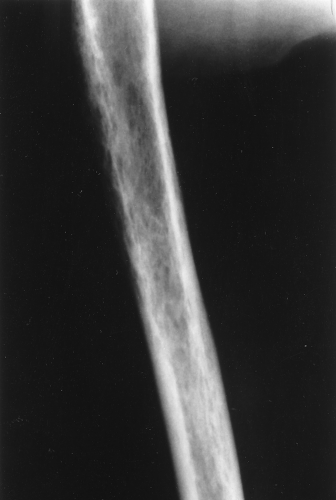
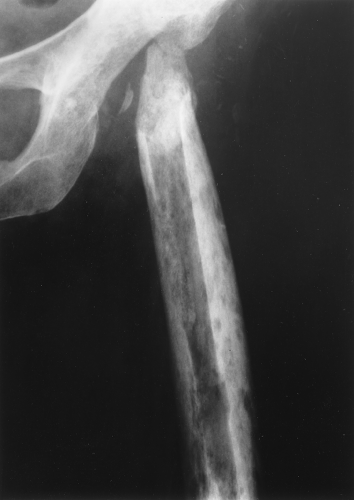
A type of osteoporosis that can be seen in a patient of any age is disuse osteoporosis. It results from immobilization from any cause, most commonly following the treatment of a fracture. The radiographic appearance of disuse osteoporosis is different from primary osteoporosis in that it occurs somewhat more rapidly and gives the bone a patchy appearance (Fig. 44.3). This is from osteoclastic resorption in the cortex causing intracortical holes. If allowed to continue with disuse, the bone would resemble any bone with marked osteoporosis, that is, severe cortical thinning.
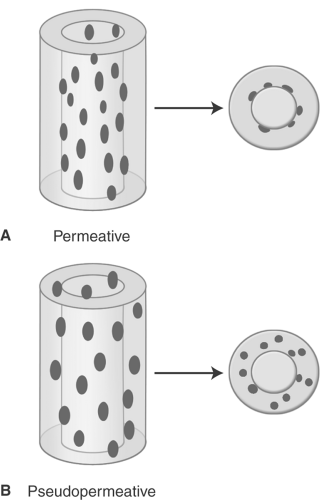
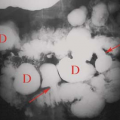
Occasionally, aggressive osteoporosis from disuse can mimic a permeative lesion such as an Ewing sarcoma or multiple myeloma because of the multiple cortical holes that project over the medullary space, thus resembling a medullary permeative process (Fig. 44.4). The way to differentiate a true intramedullary permeative process from an intracortical process such as osteoporosis is to observe the cortex and see whether it is solid or riddled with holes (Fig. 44.5). If the cortex is solid, one can assume the permeative process is emanating from the medullary space (Fig. 44.6); if the cortex has multiple small
holes, assume the permeative pattern is from the cortical process. I call a permeative appearance that is secondary to cortical holes a “pseudopermeative” process to distinguish it from a true permeative process (1).
holes, assume the permeative pattern is from the cortical process. I call a permeative appearance that is secondary to cortical holes a “pseudopermeative” process to distinguish it from a true permeative process (1).
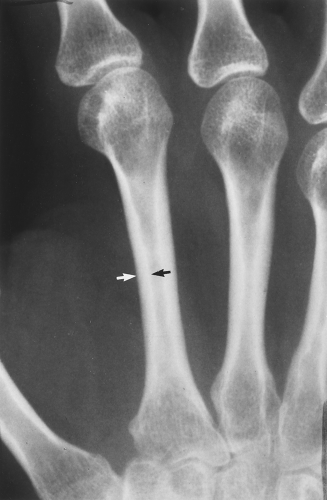 Figure 44.1. Normal Mineralization. The cortical width (arrows) at the midsecond metacarpal in this patient with normal mineralization is greater than one-third of the total width of the metacarpal. |
Other causes for a pseudopermeative process include hemangioma and radiation. A hemangioma can cause cortical holes in two ways: from focal hyperemia causing focal osteoporosis or by the blood vessels themselves tunneling through the cortex (Fig. 44.7). Radiation can cause cortical holes in bone and mimic a permeative pattern because of the death of cortical osteocytes, which can result in large lacunae in the cortex (Fig. 44.8). The cortical holes from radiation can be large, in which case they would not be confused with a true permeative process, but they can also be small and resemble an aggressive lesion.
If a permeative lesion is found, the differential diagnosis is usually an aggressive process such as Ewing sarcoma, infection, or eosinophilic granuloma in a young person (<30 years of age) or multiple myeloma, metastatic carcinomatosis, or primary lymphoma of bone in an older patient. If, however, the permeative pattern is a result of cortical holes, that is, a pseudopermeative pattern, the differential diagnosis is considerably less sinister: aggressive osteoporosis, hemangioma, or radiation changes. This differential diagnosis does not arise often but is very useful when it does (Table 44.1).
Osteomalacia
Osteomalacia is the result of too much nonmineralized osteoid. The most common cause is renal osteodystrophy. The radiographic findings are almost identical to those of osteoporosis, and, for the most part, the two disorders are indistinguishable. The only finding that is pathognomonic for osteomalacia is a Looser fracture, which is a fracture through large osteoid seams (Fig. 44.9). They are extremely uncommon but tend to occur in the femur, pelvis, and scapula.
In children, osteomalacia is called rickets. It causes the epiphyses to become flared and irregular and the long bones to undergo bending from the bone softening (Fig. 44.10). As in adults, the most common cause is renal disease, although other causes such as biliary disease and dietary insufficiencies are occasionally seen.
Table 44.1 Differential Diagnosis of Pseudopermeative Pattern | |||
|---|---|---|---|
|
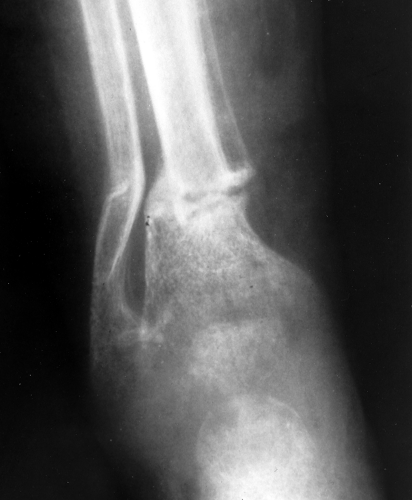 Figure 44.9. Looser Fractures in Osteomalacia. A horizontal fracture of the tibia and the fibula is present in this child with osteomalacia (rickets). Fractures of this type are called Looser fractures and are virtually pathognomonic for osteomalacia; however, they are rarely seen.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|