formation, as reflected in excessive urinary uric acid excretion (more than 600 mg/day) measured while the patient is maintained on a standard purine-free diet. Increased production can also be seen in gout secondary to myeloproliferative disorders associated with increased destruction of cells and result in increased breakdown of nucleic acids. Decreased excretion occurs in primary gout in patients with a dysfunction in the renal tubular capacity to excrete urate and in patients with chronic renal disease. In most patients, however, there is evidence of both: uric acid overproduction and diminished renal excretion of uric acid.
Table 7.1 CLINICAL AND IMAGING HALLMARKS OF METABOLIC, ENDOCRINE, AND CRYSTAL DEPOSITION ARTHROPATHIES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 Figure 7.1 ▪ Pathology of gouty arthritis. A: Gross specimen of an amputated finger from a patient with gout shows the large chalky white deposits of monosodium urate crystals. B: Sagittal section of the specimen shows the extent of the tophaceous deposit and bone destruction. C: The radiograph of the specimen shows large gouty tophi and articular bone destruction. (From Bullough PG. Atlas of Orthopedic Pathology with Clinical and Radiologic Correlation. 2nd ed. New York, NY: Gower Medical Publishing; 1992, Figs. 11.33, 11.34, and 11.35, p. 11.12.) |
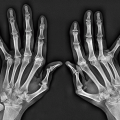
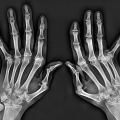
helps differentiate this condition from rheumatoid arthritis. The reason for the absence of osteoporosis is that the duration of an acute gouty attack is too short to allow the development of the disuse osteoporosis so often seen in patients with rheumatoid arthritis. If erosion involves the articular end of the bone and extends into the joint, part of the joint is usually preserved (Fig. 7.10A, see also Fig. 7.5). Unlike rheumatoid arthritis, periarticular and articular erosions are asymmetric in distribution (Fig. 7.11). In chronic tophaceous gout, monosodium urate crystal deposit in and around the joint is seen, creating dense masses in the soft tissues called tophi, which frequently exhibit calcifications (Figs. 7.12, 7.13, 7.14, 7.15, see also Figs. 7.1 and 7.2). Characteristically, tophi are randomly distributed
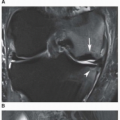
and are usually asymmetric; if they occur in the hands or feet, they are more often seen on the dorsal aspect (Fig. 7.16). Currently, dual-energy CT (DECT) color-coded images can accurately depict gouty tophi (Figs. 7.17, 7.18, 7.19, 7.20, see also Figs. 2.27 and 2.28). DECT has ability to extract information and characterize the chemical composition of material according to the differential x-ray photon energy-dependent attenuation of compounds being examined at two different energy levels. Reported sensitivity of this technique varies between 78% and 100% and specificity between 89% and 100%. Magnetic resonance imaging is also an effective way to detect articular and soft tissue abnormalities of gouty arthritis. Tophaceous gouty deposits exhibit a wide spectrum of signal intensities characteristics, which reflects their variable composition and relative proportion of protein, fibrous tissue, crystals,
and hemosiderin. Most lesions are isointense relative to muscle on T1-weighted images, and low-to-intermediate heterogeneous signal intensity on proton density-weighted and water-sensitive (IR, T2) sequences (Fig. 7.21). There is strong enhancement following intravenous injection of gadolinium, although contrast enhancement of the tophus is variable and depends on the vascularity of the affected synovium and surrounding granulation tissue (Fig. 7.22). Concomitant enhancement of adjacent tendon sheaths, ligaments, muscles, and bone marrow may also be present, reflecting intense inflammatory reaction.
 Figure 7.2 ▪ Histopathology of gouty arthritis. A: Low-power photomicrograph of a portion of the joint shown in Figure 7.1 depicts the erosion of the bone and articular cartilage by an amorphous material (H&E, original magnification ×2.5). B: On slightly higher magnification observe bluish amorphous material surrounded by bundles of dense collagenized tissue and inflammatory cells (H&E, original magnification ×4). C: Same field examined by polarized light demonstrates the birefringence of the crystalline material (H&E, polarize light, original magnification ×4). D: On the higher magnification, note a thin layer of mononuclear and giant cells surrounding the amorphous crystalline deposit, with an occasional sprinkling of chronic inflammatory cells (H&E, polarized light, original magnification ×25). (From Bullough PG. Atlas of Orthopedic Pathology with Clinical and Radiologic Correlation. 2nd ed. New York, NY: Gower Medical Publishing; 1992, Figs. 11.37, 11.38, and 11.39.) |
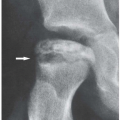
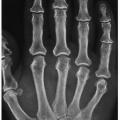
 Figure 7.6 ▪ Gouty arthritis. Typical para-articular erosions in the distal inter-phalangeal joint of the index finger exhibiting an “overhanging edge” are associated with a large tophus. |
 Figure 7.9 ▪ Gouty arthritis. Radiograph of the index finger of a 65-year-old man shows the intraosseous tophi within the middle and distal phalanges. |
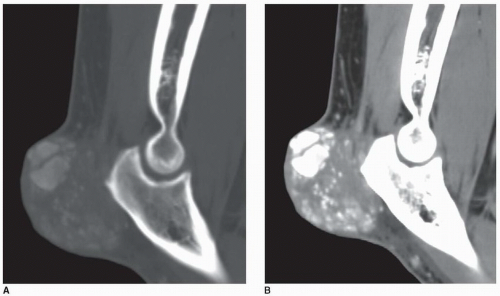
 Figure 7.20 ▪ Dual-energy CT of tophaceous gout. A: Long-axis CT image of the right foot of a 71-year-old man (same patient as shown in Fig. 7.7A) shows a nonspecific low-attenuation mass in the region of the second toe. Long-axis (B) and sagittal (C) reformatted dual-energy color-coded CT images identify the mass as being a large tophus containing monosodium urate crystals (green area). In addition, several smaller tophi are identified at the site of Lisfranc joint and at the site of Achilles tendon attachment to the calcaneus. Three-dimensional reconstructed dual-energy CT color-coded images viewed from the plantar (D) and medial (E) aspects of the foot better demonstrate the spatial distribution of the urate tophi. |
production, such as xanthine oxidase inhibitors (allopurinol or febuxostat), and medications that improve removal of uric acid from the body (probenecid), are used to prevent complications of gout. Most recently, rheumatologists reported that urate-lowering therapy using pegloticase, a pegylated mammalian (porcine-like) recombinant uricase, resulted in reduction of the gouty tophus size both at subcutaneous sites and within the joints. However, ACP recommends that clinicians should discuss benefits, harms, costs, and individual preferences with patients before initiating urate-lowering therapy in patients with recurrent gout attacks. This includes the risk of Stevens-Johnson syndrome in patients receiving allopurinol.
Table 7.2 MOST COMMON CAUSES OF CHONDROCALCINOSIS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree