Colonic Obstruction
Mechanical large bowel obstruction is four to five times less common than small bowel obstruction and differs significantly in terms of cause ( Table 62-1 ), pathophysiology, therapy, and prognosis. Colon obstruction is most often the result of a neoplasm ( Table 62-2 ), whereas most small bowel obstructions are caused by adhesions. A number of extracolonic disease processes, including gynecologic diseases, can secondarily involve the large bowel, leading to obstruction or formation of strictures and fistulas.
| Intrinsic Defects | Extrinsic Defects |
|---|---|
| Neoplasms | Volvulus |
| Benign | Secondary |
| Malignant | Primary |
| Inflammatory | Hernias |
| Diverticulitis | Internal |
| Ulcerative colitis | External |
| Crohn’s disease | Adhesions |
| Amebiasis | Mass compression |
| Tuberculosis | Carcinomatosis |
| Intussusception | Abscess |
| Obturation | Pregnancy |
| Gallstones | Cysts |
| Foreign bodies | Pancreatitis |
| Meconium | Endometriosis |
| Medications | |
| Enteroliths | |
| Bezoars | |
| Worms | |
| Congenital | |
| Atresia | |
| Stenosis | |
| Imperforate anus | |
| Cysts and duplications | |
| Miscellaneous | |
| Post-traumatic events | |
| Pneumatosis intestinalis |
| Cause | Incidence (%) |
|---|---|
| Carcinoma | 55 |
| Volvulus | 11 |
| Diverticulitis | 9 |
| Extrinsic cancer | 8 |
| Adhesions | 4 |
| Impaction | 3 |
| Hernia | 2 |
| Intrinsic | 4 |
Pathophysiology
When colonic obstruction is caused by diverticulitis or cancer, symptoms are usually subacute or chronic. Swallowed air proximal to the obstruction causes dilation, but third spacing of fluid in the gut lumen characteristic of small bowel obstruction is not seen. Strangulation rarely occurs, except in occasional cases of volvulus. Colonic response to the mechanical obstruction depends on the competency of the ileocecal valve ( Fig. 62-1 ). The small bowel serves to decompress the colon when the valve is incompetent. A closed loop obstruction develops when the valve is competent because the colon cannot decompress.

The cecum has the largest diameter of the colon, and therefore its wall develops the highest tension, according to Laplace’s law (wall tension = intraluminal pressure × radius). The increased pressure may cause separation of the muscle fibers, leading to cecal diastatic perforation. Dissection of air into the wall results in pneumatosis, which may precede frank perforation. The risk of perforation increases when the cecum reaches a diameter of 9 to 12 cm. The duration and rapidity of onset of the distention are also important. The intraluminal pressure needed to produce perforation is between 20 and 55 mm Hg. Ischemia and bacterial overgrowth also play a role in cecal perforation, and the systemic effects seen with strangulating obstruction.
Clinical Findings
Because most colonic obstructions are caused by cancer, patients are usually older adults and have symptoms related to tumor location. Signs and symptoms are often insidious with right colon lesions because the lumen is large and the contents are semiliquid. These patients often present with pain, a palpable mass, and anemia. Left-sided lesions cause progressive constipation and, ultimately, obstipation, with abdominal distention and pain. If the ileocecal valve is incompetent, retrograde decompression produces the gradual onset of distention and, eventually, feculent vomiting. Lesions occurring at the ileocecal valve or ileocolic intussusception cause more acute symptoms of small bowel obstruction—abdominal pain, distention, vomiting, and obstipation. Patients with volvulus may develop pain and distention rapidly if a closed loop obstruction and bowel ischemia are present. On physical examination, an abdominal mass may be present (e.g., advanced right-sided colon cancer) and distention may be most marked in one region (e.g., the left upper quadrant in cecal volvulus). Bowel sounds are often hyperactive, particularly with superimposed small bowel obstruction. Marked tenderness or rebound suggests perforation or strangulation.
Radiologic Approach to Suspected Large Bowel Obstruction
Radiography
Abdominal radiographs obtained in the supine and erect or left lateral decubitus positions are often the initial means of imaging patients with suspected obstruction. These radiographs may confirm the diagnosis, locate the site of obstruction and, in some cases, identify the nature of the obstructing lesion. The colon is usually dilated proximal to the obstruction; however, if the ileocecal valve is incompetent, the appearance may mimic that of small bowel obstruction. The radiographic findings on plain radiographs in bowel obstruction are discussed in detail in Chapter 10 .
Contrast Enemas
If the plain abdominal radiographs show convincing evidence of large bowel obstruction caused by volvulus or ileocolic intussusception, a contrast enema, preferably with a water-soluble medium, is the next step in patients with suspected large bowel obstruction. Although barium is inherently a better contrast agent, it may interfere with future studies, such as computed tomography (CT) or colonoscopy. If the patient proves to have a pseudo-obstruction rather than true obstruction, the barium may remain within the colon for days. A water-soluble enema can also be therapeutic in patients with fecal impactions. Because oral barium can inspissate proximal to a partial or complete large bowel obstruction, it is important to exclude an obstructing colonic lesion before the small bowel is examined with barium.
Computed Tomography
CT is now the imaging modality of choice for patients with known or suspected obstruction. It is the best noninvasive means of answering the following key questions:
- •
Is the bowel obstructed?
- •
What is the level of the obstruction?
- •
What is the cause of the obstruction?
- •
Is the obstruction simple or closed loop?
- •
Are ischemic changes present?
- •
Does the patient need immediate surgery, or should a trial of conservative therapy be attempted first?
CT is most valuable when there are systemic signs suggesting infection, bowel infarction, or an associated palpable mass. CT identifies bowel obstruction as distended bowel loops seen proximal to collapsed loops and can reveal the cause of obstruction, such as tumor, volvulus, diverticulitis, or appendicitis. The transitional zone should be carefully evaluated for masses.
Ultrasonography and Magnetic Resonance Imaging
Although CT is the best examination for evaluating patients with suspected colon obstruction, ultrasonography and magnetic resonance imaging (MRI) can also determine the level and cause of obstruction, particularly in children and pregnant women.
Major Types of Colonic Obstruction
Carcinoma of the Colon
Large bowel obstruction is caused by intrinsic colon carcinoma in approximately 55% of cases. Almost 20% of colon cancers are complicated by some degree of obstruction; 5% to 10% are complicated by complete obstruction, which requires emergent surgical intervention. The mortality rate is high (10%-30%) in patients requiring emergency surgery, regardless of the site of tumor. This is a reflection of advanced tumor stage, advanced patient age, and associated cardiorespiratory disease, malnutrition, sepsis, and surgical stress. Mortality is even higher if there is concurrent perforation. Patients who present with obstructing colon carcinoma have low curability and survival rates because of advanced disease at the time of diagnosis.
The sigmoid colon is the most common site of obstructive colon cancer because of its relatively narrow diameter and solid fecal contents. The location of obstruction in one series was as follows: cecum, 11%; right colon, 5%; hepatic flexure, 3%; transverse colon, 11%; splenic flexure, 12%; descending colon, 10%; sigmoid colon, 35%; and rectum, 13%. The ratio of obstructing carcinomas to total tumors is similar in the right ( Fig. 62-2 ) and left colon but lower for rectal lesions. Tumors of the transverse colon and splenic flexure are at particular risk for obstruction before clinical detection. The risk of developing obstruction in tumors at a particular site has been estimated as 50% for splenic flexure lesions, 25% for right- and left-sided ones, and 6% for rectal tumors. Perforation occurs in 3% to 8% of patients with malignant obstruction. The most common site of perforation is the cecum rather than adjacent to the tumor.

The clinical presentation of obstructive colon cancer depends on the location, duration, and completeness of obstruction, as well as on the competency of the ileocecal valve. Tumors located near the ileocecal valve can produce symptoms suggesting a distal small bowel obstruction. Some right colon tumors act as lead points for colocolic intussusception; others may perforate, causing abscess formation and obstruction. Left-sided colon tumors tend to obstruct in an annular fashion or by a polypoid lesion that reaches a size sufficient to obstruct the lumen. The rectum is not a common site of obstruction because of its greater diameter and distensibility and because rectal cancers often cause rectal bleeding, prompting earlier medical attention. The usual treatment of obstructive colon cancer is surgical resection or diverting colostomy; however, expandable metallic stents can be used for palliation or to allow bowel cleansing before surgical resection.
Diverticulitis
Large bowel obstruction is the result of diverticular disease in about 12% of cases. Diverticulitis can cause small and large bowel obstruction. Partial colonic obstruction can complicate acute diverticulitis as a consequence of edema and pericolic inflammation or abscess formation. High-grade obstruction is uncommon; it is far more frequently caused by carcinoma of the colon. Usually, obstruction follows recurrent attacks of diverticulitis, with marked fibrosis of the colon wall leading to narrowing and eventually stricture formation. The site of obstruction is usually in the sigmoid colon, near the site of inflammation. Obstruction of the transverse or right colon attributable to diverticulitis is rare.
Clinically, patients with sigmoid diverticulitis complain of left lower quadrant pain, fever, and abnormal bowel habits. It is important for these symptoms to be differentiated from those of carcinoma of the colon, an often difficult task clinically and radiologically ( Fig. 62-3 and Table 62-3 ). Symptoms of sigmoid colon cancer are usually more insidious, with rectal bleeding, constipation, and weight loss. A more complete discussion of diverticular disease can be found in Chapter 55 .

| Diverticulitis | Carcinoma |
|---|---|
| Spastic bowel | Normal adjacent bowel |
| Cone-shaped edges | Sharp margins, shelflike |
| Long segments | Short segments |
| Mucosa preserved | Mucosa destroyed |
| Variable constriction between examinations | Progressive obstruction |
| Diverticula present | Occasional diverticula |
| Obstruction without tumor | Obstruction with tumor |
Volvulus
Volvulus refers to torsion or twist of an organ on a pedicle to a degree sufficient to cause symptoms. Large bowel volvulus ( Fig. 62-4 ) accounts for about 10% of colon obstructions and can affect the sigmoid colon ( Fig. 62-5 ), cecum ( Figs. 62-6 and 62-7 ), transverse colon and, rarely, the splenic flexure. Symptoms are caused by narrowing and obstruction of the gut, strangulation of the blood vessels, or both.




The major predisposing factors necessary for colonic volvulus are a segment of redundant mobile colon on a mesentery or mesocolon and a fixed point around which rotation can occur. The sigmoid colon ( Fig. 62-8 ) is therefore the most frequent site of colonic volvulus, especially in patients older than 60 years. Sigmoid volvulus is not caused by a congenital defect but by dietary and behavioral factors, including increased fiber in the diet, which increases the bulk of stool and elongates the colon. Cecal or ascending colon volvulus occurs in patients with a congenital defect in attachment of the right colon or postpartum ligamentous laxity and a mobile cecum. Anything that causes colon distention, including pseudo-obstruction, distal tumor, endoscopy, enemas, and postoperative ileus, may precipitate cecal volvulus in susceptible individuals. Transverse colon volvulus occurs in patients with a redundant transverse colon on a long mesentery, and splenic flexure volvulus occurs in patients with deficiency of the normal attachments of the splenic flexure to the posterior peritoneal wall, usually as a result of prior surgery. The frequency of colon volvulus is as follows: sigmoid colon, 50% to 75%; cecum, 25% to 40%; and transverse colon, 0% to 10%. A rare cause of combined colon and small bowel obstruction is the ileosigmoid knot. A detailed discussion of colonic volvulus and its conventional radiographic features can be found in Chapter 10 .

On CT, a whirl sign has been described in volvulus. The whirl is constituted by the afferent and efferent limbs leading into the volvulus. Tightly twisted mesentery and bowel compose the central portion of the whirl. The progressive tapering of the two limbs leading to the twist on CT corresponds to the so-called beak seen on barium enema. The distended and redundant sigmoid colon may overlie the liver and extend cephalad to the transverse colon (the so-called northern exposure sign.
Adult Intussusception
Intussusception is defined as the invagination of one segment of the gastrointestinal (GI) tract into an adjacent one ( Fig. 62-9 ). It accounts for 80% to 90% of bowel obstruction in infants and children and ranks second only to appendicitis as the most common cause of an acute abdominal emergency in children. In children, intussusception usually begins in the distal ileum, and in 90% of cases the cause is idiopathic. A complete discussion of childhood intussusception can be found in Chapter 121 .

In contrast, intussusception in adults accounts for only 1% to 3% of mechanical intestinal obstruction, and a demonstrable cause is found in 80% of adult cases. Colonic intussusception is usually caused by a primary colon cancer, whereas small bowel intussusception is generally related to a benign tumor and less often to a malignancy, usually a metastatic lesion. Postoperative intussusceptions usually occur in the small bowel and are related to a number of factors, such as suture lines, ostomy closure, adhesions, long intestinal tubes, bypassed intestinal segments, submucosal edema, abnormal bowel motility, electrolyte imbalance, and chronic dilation of the bowel.
Benign lesions that can serve as lead points in colonic intussusception include adenomatous polyps, lipomas, gastrointestinal stromal tumors ( Fig. 62-10 ), appendiceal stump granulomas, and villous adenomas of the appendix. The normal appendix may transiently intussuscept, although clinically significant appendiceal intussusception usually occurs in the setting of appendiceal inflammation, infestation, neoplasm, or endometriosis deposition. A more complete discussion of diseases of the appendix can be found in Chapter 56 . Colonic intussusception has also been reported as a complication of eosinophilic colitis, epiploic appendagitis, and pseudomembranous colitis.

In children, the diagnosis is suggested by the presence of an abdominal mass and passage of blood per rectum. Because adult intussusception is often chronic and relapsing, the diagnosis is suggested by recurrent episodes of subacute obstruction and variable abdominal signs. During the height of an attack, a palpable mass may be present but may disappear completely when the patient is reexamined several hours later, by which time the symptoms have resolved. In infants and young children, hydrostatic or pneumatic intussusception reduction should be attempted and may be definitive therapy because the cause is usually idiopathic. In adults, the high incidence of organic lesions, often malignant, precludes this approach.
If the intussusception is of the ileocolic or colocolic variety, the pathognomonic crescent sign may be seen. This sign is produced when the intussuscipiens invaginates into the intussusceptum and stretches the outer wall. Intraluminal gas trapped between the two intestinal surfaces can appear as a semilunar lucency lacking haustral septa or valvulae conniventes. This lucent crescent is wider than normal bowel in diameter and is often superimposed on a round soft tissue density representing the mass created by the intussusception. A more central and less distinct lucency may be seen, representing gas trapped in the lumen of the intussuscipiens. On supine views, the transverse colon gas shadow is often absent because this segment of colon is usually displaced distally.
The classic appearance of intussusception on barium studies is the coil spring appearance as contrast is trapped between the intussusceptum and intussuscipiens ( Fig. 62-11 ).

Ultrasound shows a target-like lesion ( Fig. 62-12 ), in which the hypoechoic halo is produced by the mesentery and edematous wall of the intussuscipiens, and the hyperechoic center is produced by multiple interfaces of compressed mucosal, submucosal, and serosal surfaces of the intussusceptum. Multiple concentric rings, best seen on transverse scans, are also characteristic. The corresponding appearance on longitudinal scans is that of multiple, thin, parallel, hypoechoic, and echogenic stripes.

On CT, intussusceptions appear as three different patterns, which reflect their severity and duration: (1) the target sign; (2) a sausage-shaped mass, with alternating layers of low and high attenuation; and (3) a reniform mass. Pathophysiologically, the target is the earliest stage of intussusception. As it progresses, a layering pattern develops, with alternating low-attenuation (mesenteric fat) and high-attenuation areas (bowel wall), ( Fig. 62-13 ). If intussusception is untreated, edema and mural thickening develop. An intussusception with a reniform appearance is caused by severe edema and vascular compromise, which constitutes a surgical emergency. Intussusception is almost invariably associated with acute intestinal obstruction or partial and recurrent obstruction, air-fluid levels, and proximal bowel distention. The mesenteric arcade associated with the intussuscipiens may show traction. If infarction occurs, this mass may be surrounded by intraperitoneal fluid, edema, hemorrhage in the mesentery, and even perforation. CT can diagnose lipoma as the lead point; however, with varying degrees of infarction and fat necrosis, lipomas may have an atypical appearance.

Adhesions
Adhesions are the result of inflammation that heals with fibrosis. They are usually the result of surgical trauma but may be congenital. Adhesional large bowel obstruction is unusual because the colon is characteristically fixed and of large caliber and has thick walls. The small bowel, in contrast, has an inherently small caliber and high degree of mobility and is therefore very prone to obstruction by adhesions. A more complete discussion of small bowel obstruction can be found in Chapter 46 . The mobile redundant portions of the colon are most likely to be involved. Adhesive obstructions of the right colon, transverse colon, and sigmoid colon have been reported.
Folding of the cecum on itself, the cecal bascule, often occurs at the site of an adhesive band. The ascending colon can be obstructed by congenital bands or adhesions caused by inflammatory changes after colonoscopy and polypectomy. Inflammation of the appendices epiploicae can rarely cause obstruction in the rectosigmoid, and ischemia and inflammation of the greater omentum can cause obstruction in the transverse colon. On barium enema, adhesions can appear as an area of circumferential narrowing, usually short, with intact mucosa, or as a smooth, broad-based filling defect. They may produce partial or complete colonic obstruction.
Hernias
Hernias cause large bowel obstruction less often than small bowel obstruction because of the relatively fixed nature of the colon and its larger caliber. Inguinal, femoral, umbilical, spigelian, incisional, lumbar, and diaphragmatic hernias (congenital or post-traumatic) can all contain colon and cause bowel obstruction. Internal hernias, such as through the foramen of Winslow, can contain colon and cause obstruction as well. The diagnosis may be made by radiography, barium enema, or CT. A complete discussion of hernias can be found in Chapter 112 .
Obturation Obstruction
The terminal ileum is the narrowest portion of the gut and, as a result, is the most common site of obturation obstruction. The sigmoid colon, measuring 2.5 cm in diameter, only slightly larger than the distal ileum, is the narrowest portion of the colon, followed by the hepatic and splenic flexures. These are the most likely points of colonic impaction of an intraluminal object.
Of patients with gallstone ileus, 3% to 5% have colonic obstruction. In these patients, the gallstone most often bypasses the ileocecal valve through a cholecystocolic fistula and usually impacts in the sigmoid colon, which may be narrowed by prior diverticulitis.
Bezoars, foods, and enteroliths usually do not obstruct the colon unless there is a stricture. Patients with cystic fibrosis (see Chapter 118 ) tend to form large cecal masses of inspissated feces that can cause the meconium ileus equivalent syndrome.
Sufficient amounts of various medications or diagnostic agents can cause colonic obturation obstruction. Antacid impactions containing nonabsorbable aluminum hydroxide antacid gels, given to prevent hyperphosphatemia, can develop in the right colon of patients with renal failure. These impactions can be treated with orally or rectally administered hyperosmolar diatrizoate meglumine (Gastrografin).
Barium retained in the colon for several days is desiccated and can form impactions in any part of the colon, usually the proximal portion. These impactions can also be treated by hyperosmolar Gastrografin, which stimulates peristalsis and draws fluid into the bowel. Foreign bodies entering via an oral or transanal route can cause obstruction or perforation.
Older, inactive, debilitated, and mentally challenged patients, drug addicts in methadone maintenance programs, transplantation and hemodialysis patients, and those with adult Hirschsprung’s disease are at risk for developing fecal impaction. The most common sites are the rectum (70%) and sigmoid colon (20%). A fecaloma or stercoroma is a mass of fecal material that develops the characteristics of an intraluminal tumor. These are composed primarily of an accumulation of undigested food forming a nucleus around which fecal material accumulates. Obstruction, volvulus, stercoral ulceration, perforation, rectal prolapse, and rectal fissure are potential complications of fecalomas.
Strictures
Colonic strictures can result from a variety of disorders ( Boxes 62-1 to 62-3 ). These strictures often cause chronic obstructive symptoms and may cause acute obstruction from the presence of impacted feces or as a result of obturation in the stricture by a foreign object.
Inferior mesenteric artery ligation, thrombosis, or embolus
Superior mesenteric artery ligation, thrombosis, or embolus
Cardiac arrhythmia
Congestive heart failure
Shock
Digitalis toxicity
Collagen vascular disease
Strangulated hernia
Oral contraceptives
Polycythemia vera
Trauma
Diabetes
Amyloidosis
Radiation
Lifestyle
Inadequate fiber
Little food
Repressing or ignoring urge to defecate
Immobility
External Factors
Drugs (including opiates, anticholinergics, antidepressants, anticonvulsants)
Endocrine and Metabolic Factors
Hypothyroidism
Hypercalcemia
Porphyria
Neurologic
Parkinson’s disease
Multiple sclerosis
Spinal lesions
Damage to sacral parasympathetic nerves
Autonomic neuropathy
Autonomic failure
Psychologic Factors
Depression
Eating disorders (e.g., anorexia nervosa)
Obsession about “inner cleanliness”
Denied bowel actions
Gastrointestinal Tract
Obstruction
Aganglionosis (Hirschsprung’s disease, Chagas’ disease)
Myopathy
Neuropathy
Systemic sclerosis
Megarectum and Colon
Anal atresia or malformation
Hereditary internal anal sphincter myopathy
Anal stenosis
Weak pelvic floor
Large rectocele
Internal intussusception
Anterior mucosal prolapse
Prolapse
Solitary rectal ulcer
Other Causes
Pelvic lipomatosis, in which there is benign fatty tissue infiltration into the perirectal and perivesical spaces, usually causes straightening, narrowing, and rigidity of the rectum but occasionally can cause obstruction. Pregnancy, mesenteric cysts, wandering spleen, retroperitoneal tumors, retractile mesenteritis, and retroperitoneal fibrosis are rarer causes of large bowel obstruction.
Extracolonic Diseases Involving the Colon
Benign and malignant extrinsic disease processes can involve the colon. Endometriosis, ovarian carcinoma, other benign and malignant gynecologic diseases, pancreatic disease, prostate carcinoma, and other abdominal malignancies can present as colon involvement. This involvement may cause obstruction and can mimic primary colon pathology. Obstruction usually occurs in the pelvis, where the colon is fixed and confined by the bony pelvis. The transverse colon is another common site of involvement because of its omental and mesenteric attachments (see Chapter 108 ). Barium enema, CT, and endoscopic ultrasonography may be helpful in the assessment of colonic wall invasion by adjacent disease processes.
Endometriosis
Endometriosis is a disease that affects 8% to 18% of women during their active menstrual years, usually between the ages of 30 and 45 years. GI tract involvement has been reported in 5% to 37% of patients. In one large retrospective review of over 7000 patients with endometriosis, 12% of the patients had gut involvement. The rectosigmoid colon was the most frequently involved site (72% of cases), followed by the rectovaginal septum (14%), small intestine (7%), cecum (4%), and appendix (3%). Rare cases of involvement of the stomach, pancreas, proximal small bowel, and transverse colon have been reported.
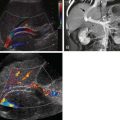
The anterior wall of the rectosigmoid colon adjacent to the pouch of Douglas is the most frequent site of GI tract involvement. Endometrial implants on the bowel are usually extrinsic or serosal but may be intramural or, rarely, intraluminal. Implants typically appear as an extrinsic serosal mass effect with mucosal preservation on double-contrast barium enema studies ( Fig. 62-14 ). This appearance, although characteristic of endometriosis, is not specific because other extrinsic processes in the cul-de-sac, such as drop metastases or an abscess, can produce an identical appearance. Serosal spiculation and fine crenulations of the mucosa can usually be identified when there is involvement of the bowel wall. Less frequently, manifestations of endometriosis on barium enema are a polypoid mass extending into the lumen of the colon, stricture, or short annular lesion. These cases may be difficult to distinguish clinically and radiographically from carcinoma. Rarely, endometriosis presents as acute obstruction, GI bleeding, or perforation, necessitating resection. Endometriomas usually appear as solid or complex cystic masses on ultrasound or CT, but the appearance is not specific and may be indistinguishable from a neoplasm or abscess. MRI (see Fig. 62-14 ) is more sensitive and specific for detection of hemorrhage within these masses.

Malignant Gynecologic Tumors
Malignant gynecologic neoplasms spread within the peritoneal cavity by direct invasion, intraperitoneal seeding, hematogenous metastasis, and lymphatic extension. Intraperitoneal seeding of malignant cells is dependent on the natural pathways of flow of ascitic fluid as demonstrated by Meyers. The characteristic patterns of spread vary depending on the origin of the primary tumor. See Chapter 109 for a more complete discussion of pathways of abdominal and pelvic spread of disease.
Ovarian Carcinoma
Ovarian carcinoma is the fifth leading cause of cancer deaths in women; the prevalence increases with age and peaks around age 60 years. Most patients with ovarian carcinoma have relatively few symptoms, and in over 50% the disease is in an advanced stage at the time of initial presentation. Patients usually have vague abdominal symptoms and present with increasing abdominal girth and a large palpable mass, although some tumors are detected earlier on routine pelvic examination. Occasionally, intestinal symptoms or colonic obstruction may be the first manifestation of ovarian cancer.
Ovarian carcinoma is the most common primary malignancy to invade the colon directly. The subperitoneal space forms a pathway for the spread of disease processes from the pelvis to the abdomen. Direct invasion from the left ovary through the sigmoid mesocolon usually involves the inferior border of the sigmoid colon. Direct invasion from the right ovary usually involves the cecum and distal ileum by means of extension through the small bowel mesentery of the ileum. Direct invasion of the colon by ovarian cancer can be readily demonstrated on barium enema, with appearances ranging from serosal spiculation with tethering and fixation to gross invasion and annular constriction to complete obstruction. Mural involvement may produce narrowing with mucosal crenulations or, in more severe cases, marked narrowing with spiculation. Annular constriction may simulate carcinoma or diverticulitis, and complete obstruction may mimic an obstructing colon carcinoma.
Ovarian carcinoma frequently spreads by intraperitoneal seeding. The colon is the portion of the GI tract most frequently involved by intraperitoneal seeding of ovarian carcinoma. Peritoneal implants, which may be multiple, are seen as extrinsic masses, often with serosal spiculation and tethering ( Fig. 62-15 ) on barium enema studies. An implant in the cul-de-sac may be indistinguishable from an abscess or endometrioma. In addition, ovarian carcinoma frequently seeds the greater omentum, producing a so-called omental cake, which can secondarily involve the transverse colon or stomach. The appearance in these cases can simulate gastric cancer with extension to the transverse colon through the gastrocolic ligament or colon cancer with extension to the stomach.

Other Gynecologic Malignancies
Cervical carcinoma spreads by direct extension to the pelvic sidewall and adjacent structures, including the rectum. Demonstration of rectal involvement is an important finding because hysterectomy is reserved for patients with tumor localized to the cervix or extension confined to the parametrium or upper two thirds of the vagina (stage IIB or less). All others are treated with radiation therapy.
Carcinoma of the endometrium, vagina, and fallopian tubes can also invade the rectosigmoid colon directly. Barium enema examination provides important information for staging and planning surgical resection of these pelvic malignancies. These tumors can also spread by peritoneal seeding or lymphatic extension. Leiomyosarcoma of the uterus, like other sarcomas, may spread by hematogenous metastasis to the gut, producing submucosal masses that can ulcerate and bleed.
Benign Gynecologic Tumors
Benign ovarian tumors, including serous and mucinous cystadenomas and teratomas, may produce an extrinsic impression on the rectosigmoid colon that is recognizable on CT and barium enema. These masses, which are purely extrinsic to the bowel, have a smooth interface with the colonic wall and are usually asymptomatic but, if very large, may produce symptoms of partial obstruction. Teratomas usually contain fat or calcification that can be detected on plain films or CT. Uterine fibroids are present in 25% to 50% of women and are the most common pelvic tumor, with a peak incidence in the fifth decade of life. Most are small and asymptomatic. Fibroids may present as vaginal bleeding if they are submucosal or may become symptomatic if they grow to a large size. Barium enema in these cases demonstrates a smooth extrinsic mass effect on the sigmoid colon, with marked displacement and stretching of the colon if the tumor is large. Rupture of an ovarian cystadenoma into the peritoneal cavity may result in pseudomyxoma peritonei.
Pelvic Inflammatory Disease and Tubo-Ovarian Abscess
Pelvic inflammatory disease has a variety of causative organisms, including Chlamydia trachomatis, Neisseria gonorrhoeae, and a number of other aerobic and anaerobic bacteria. The tubo-ovarian abscesses that result may secondarily involve the bowel and may be indistinguishable on barium enema from endometrial or metastatic implants on the serosal surface. The cul-de-sac is a frequent site of involvement. Ultrasonography, CT, or MRI shows a thick-walled fluid collection that may be difficult or impossible to differentiate from cystic neoplasm or endometrioma.
Sexually Transmitted Diseases
Lymphogranuloma venereum is a sexually transmitted disease caused by infection of lymphatic tissue by C. trachomatis . The site of primary infection is usually around the genitals, but the rectum may be the primary site of involvement. Primary infection of the rectum is usually acquired by anal intercourse, but proctitis may also result from the spread of an infected vaginal discharge to the anal canal or by lymphatic extension from inguinal lymph nodes. Patients present with inguinal adenopathy and deep rectal ulceration. The disease may progress to form a fistula, perirectal abscess, or stricture ( Fig. 62-16 ). Other sexually transmitted diseases, including N . gonorrhoeae or herpes simplex infection and syphilis, can infect the rectum and cause proctitis. Barium enema demonstrates mucosal ulceration or a granular mucosal pattern. CT typically shows nonspecific mural thickening of the rectum and anus.

Pancreatitis
Approximately 1% of patients with acute pancreatitis have significant colonic problems. These complications usually occur 10 to 21 days after disease onset and may relate to compression caused by a pancreatic phlegmon or abscess and widespread fat necrosis, or by mural thickening caused by edema and necrosis secondary to pancreatic enzymes dissecting down the transverse mesocolon or phrenicocolic ligament. Occasionally, a high-grade obstruction or perforation may occur that requires surgical intervention. For a more complete discussion of pancreatitis, see Chapter 97 .
Metastases
High-grade colonic obstruction is attributable much less often to metastatic than to primary tumors. Renal and prostate neoplasms can directly invade the colon. Carcinoma of the prostate involves the rectum in 0.5% to 11% of cases and may simulate rectal carcinoma clinically. Rectal obstruction occurs when there is direct extension through the thick, double-layered Denonvillier’s fascia or by rectal compression caused by a massively enlarged prostate gland. Gastric and pancreatic neoplasms can invade the colon directly or narrow it by serosal metastases.
Colonic Ischemia
Clinical Findings
Colonic ischemia is the most common vascular disorder of the GI tract and the most common cause of colitis in older adults. Although many conditions have been implicated in the pathogenesis of colonic ischemia (see Box 62-1 ), the cause is unclear in most patients, and no precipitating event or condition can be identified. Most patients have no major vascular occlusion, so the condition is attributed to low flow states, small vessel disease, or both. In about 20% of cases, an obstructive or potentially obstructive colonic lesion is present.
Colonic ischemia produces a wide spectrum of disease, ranging from gangrene of the colon to transient ischemic colitis. Colonic ischemia is classified as follows: (1) reversible ischemic colopathy; (2) reversible or transient ischemic colitis; (3) chronic ulcerative ischemic colitis; (4) ischemic colonic stricture; (5) colonic gangrene; or (6) fulminant universal ischemic colitis.
The underlying pathophysiology of colonic ischemia is insufficient blood supply to the bowel to meet the needs of the mucosa. The mucosa receives most of the intestinal blood flow and is most susceptible to damage. Injury may be confined to the mucosa and submucosa or may extend transmurally. The degree of damage depends on the rate of onset of the ischemic event and extent of the vascular deprivation. The outcome may be complete healing, reversible or chronic colitis, stricture formation, or gangrene. About 50% of patients fall into the first category, with mild disease that resolves in 1 to 2 weeks. The clinical course is highly variable. In one series, the outcomes were as follows: reversible hemorrhage and edema, 33%; transient or reversible colitis, 16%; reversible or persistent strictures, 12%; persistent colitis, 21%; and gangrene of perforation, 18%.
Most patients with colonic ischemia are older than 50 years and present with mild lower abdominal pain and rectal bleeding or bloody diarrhea. Those with transmural disease have more impressive findings, with peritoneal signs. The frequency of distribution of ischemic colitis is as follows: left colon (32.6%), distal colon (24.6%), right colon (25.2%), transverse colon (10.2%), and pancolitis (7.3%).The diagnosis is usually based on colonoscopy and CT results.
Pathologic Findings
Acute ischemic lesions of the colon show necrosis ( Fig. 62-17 ) of the superficial portion of the mucosa, which often spares the deeper portions of the colonic crypts. The remaining crypts typically have an atrophic or withered appearance that reveals striking cytoplasmic atypia, which may be mistaken for dysplasia. Pseudomembranes, hemorrhage into the lamina propria, and hyalinization of the lamina propria may also be seen. These lesions may regress on their own, or frank gangrene with perforation or stricture formation may occur.

The chronic phase of ischemic colitis may be more difficult to diagnose, because the only histologic findings may be areas of submucosal fibrosis and stricture, which are nonspecific.
Radiologic Findings
Radiography
Abdominal radiographs are usually nondiagnostic and are normal or show a nonspecific ileus. In a series of 41 patients with colonic ischemia, only 21% had radiographic findings suggesting ischemia. Radiographic findings in colonic ischemia include normal, nonspecific ileus, thumbprinting, transverse ridging, and fixed loops with tubulation and lack of haustra. The most characteristic finding on radiographic and barium enema examination is thumbprinting, which consists of multiple round, smooth, scalloped defects projecting into the air-filled colonic lumen ( Fig. 62-18 ). Thumbprinting is caused by submucosal bleeding, edema, or both, and usually occurs within the first 24 hours after the insult to the bowel. Experimental work has shown that thumbprinting results from extravasation of blood around small blood vessels that are reperfused after losing their integrity during the ischemic period. Thickening of the bowel wall secondary to edema and hemorrhage may be observed; this is reflected by luminal narrowing or transverse ridging. As the edema progresses or mucosal sloughing occurs, fixed rigid, tubular, ahaustral loops may develop. Rarely, pneumatosis or portal venous gas can be identified. These findings generally indicate necrotic bowel.

Barium Enema
Colonoscopy and CT are the primary means of establishing the diagnosis of ischemic colitis. The barium enema findings depend on the extent of the ischemic process, degree of damage, and stage at which the examination is performed. The hallmark of colonic ischemia is serial changes on examinations performed over days, weeks, or months.
The classic barium enema findings in colonic ischemia are thumbprinting, transverse ridging, spasm, ulceration, intramural barium, and strictures. The most consistent and characteristic finding on the barium enema examination early in the course of the disease is thumbprinting, which is seen in 75% of patients. Thumbprinting on a barium enema study corresponds to the radiographic findings of smooth, round, polypoid, and scalloped filling defects projecting into the opacified colonic lumen as a result of submucosal edema or hemorrhage ( Fig. 62-19 ). Edema and hemorrhage may be soft and compressible, and taking films with maximal distention may affect the findings. Because spasm often accompanies ischemia, maximal distention may be hard to maintain, making thumbprinting easier to see. Careful fluoroscopy with imaging during the filling stage of the examination and postevacuation radiographs should be obtained to document the presence of thumbprinting. Intraluminal pressures during single- and double-contrast examinations are fairly equivalent, so there is no advantage to performing a single-contrast study in this regard. Thumbprinting characteristically resorbs in less than 1 week but may persist for weeks in some patients. Thumbprinting, although characteristic of ischemia, may also be observed in other conditions, including inflammatory bowel disease and the infectious colitides.