Fig. 6.1
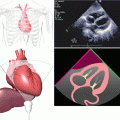
MitraClip device (Abbott Vascular)
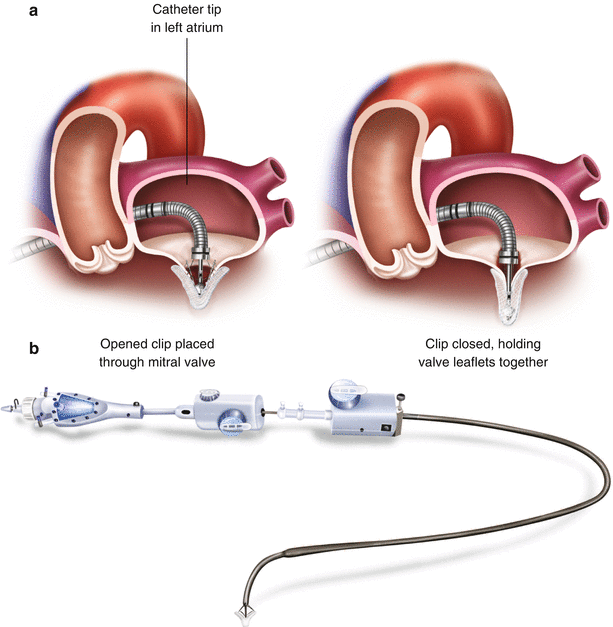
Fig. 6.2
(a) Mechanism of MitraClip. (b) Clip and guide catheter
The clip itself consists of two arms and two grippers. Using catheter guidance, the leaflets are positioned in the base of the V shape created between the gripper and clip arm. Once there is appropriate leaflet stability, the device is deployed from the catheter with the leaflet tips sandwiched between the gripper and clip arm on either side (Fig. 6.3). The goal is to deploy the clip perpendicular to the valve leaflets with complete leaflet insertion bilaterally and stable leaflet tissue medial and lateral to the clip (Fig. 6.4).
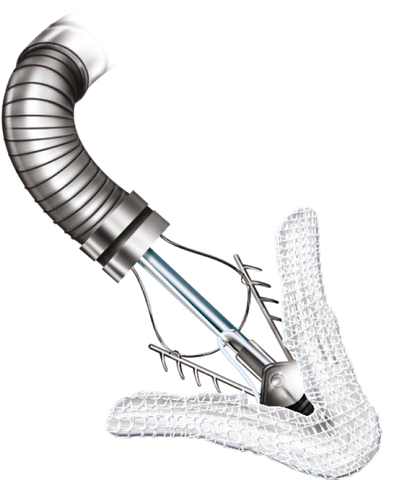
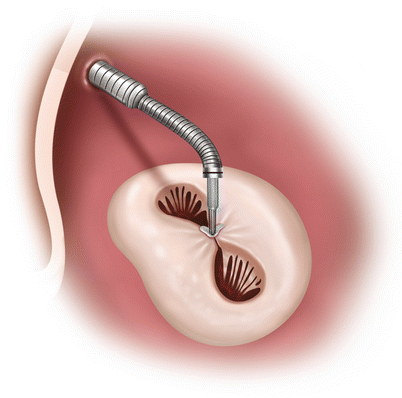

Fig. 6.3
MitraClip device, consisting of two arms and two grippers

Fig. 6.4
The MitraClip is deployed perpendicular to the valve leaflets, with complete leaflet insertion bilaterally and stable leaflet tissue medial and lateral to the clip
6.1.2 Occluder Devices
The Amplatzer Vascular Plug II from St. Jude Medical is a representative example of the current generation of occluder devices (Fig. 6.5). These products are designed for precise, catheter-guided delivery and can be recaptured and repositioned to optimize orientation prior to final deployment. They typically have a Nitinol mesh design and come in a variety of different sizes. These features make them ideally suited for percutaneous closure of mitral paravalvular leaks, thereby allowing some patients to avoid redoing of open mitral valve surgery.

Fig. 6.5
The Amplatzer Vascular Plug II (St. Jude Medical) is a representative example of the current generation of occluder devices
6.2 Case 1. Procedural Steps for MitraClip Deployment
An 82-year-old man with a past history of coronary artery bypass graft surgery, chronic obstructive pulmonary disease (COPD) on home oxygen, an abdominal aortic aneurysm, and mitral valve prolapse presented for evaluation of worsening fatigue and shortness of breath at rest in the setting of severe mitral regurgitation. Because of his significant comorbidities, he had been previously managed medically for his mitral valve disease. He was referred for MitraClip, which was performed under transesophageal guidance (Figs. 6.6, 6.7, 6.8, 6.9, 6.10, 6.11, 6.12, 6.13, 6.14, 6.15, 6.16, 6.17, 6.18, 6.19, 6.20, 6.21, 6.22, 6.23, and 6.24).
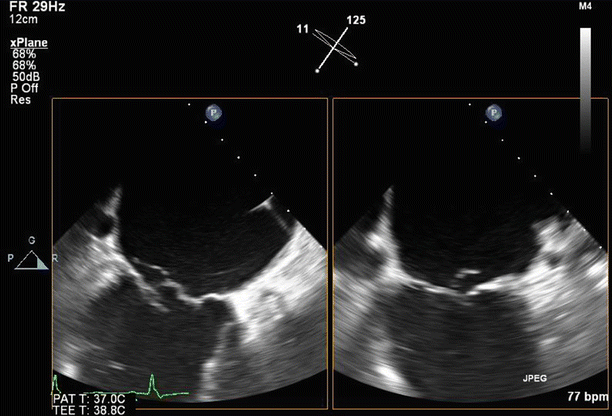
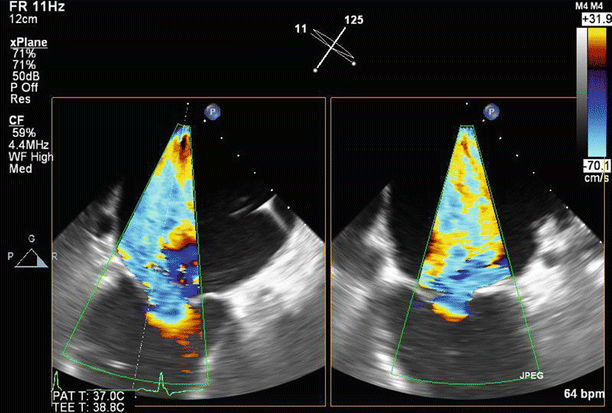
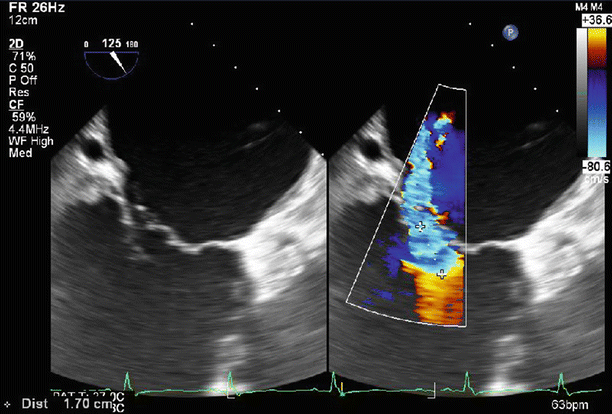
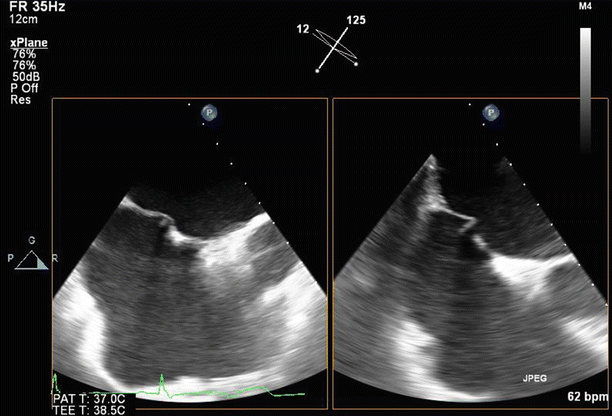
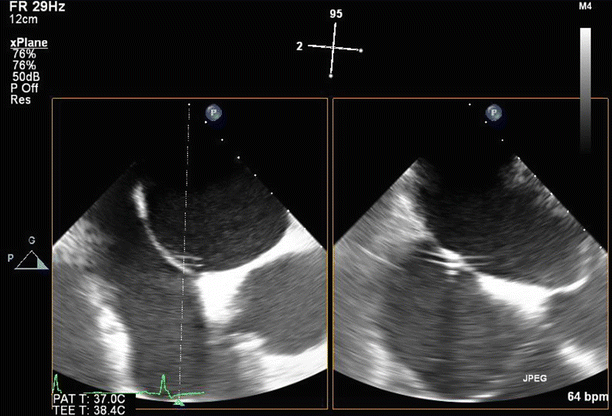
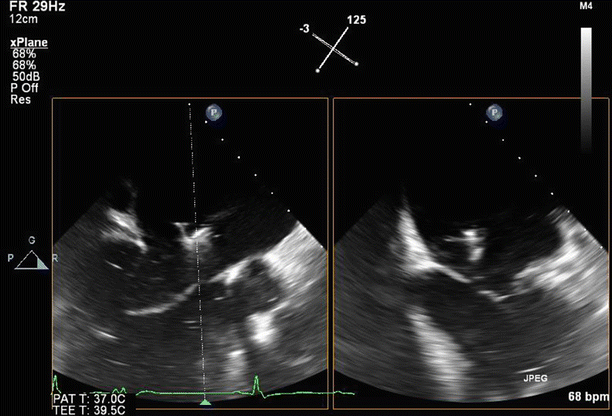
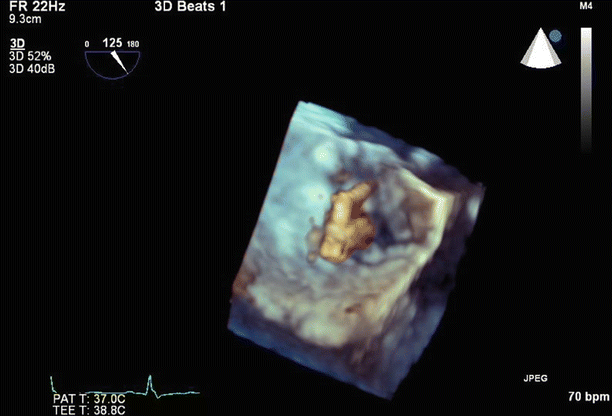
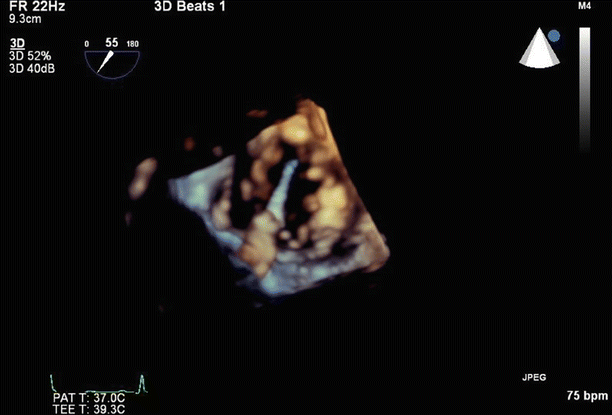
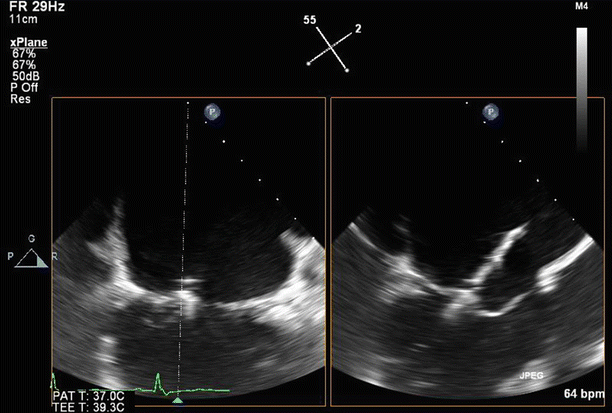
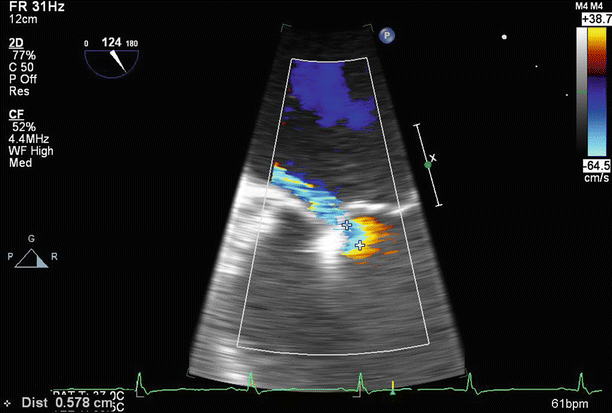
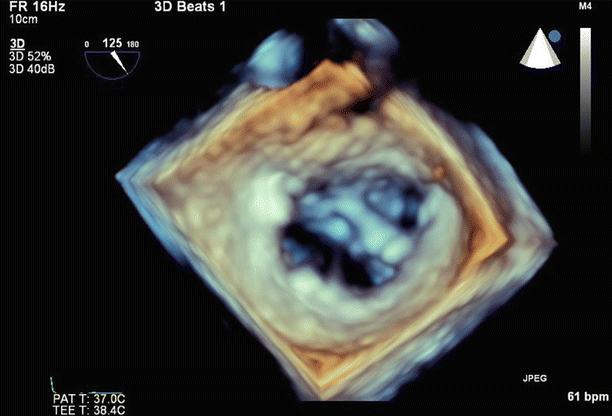
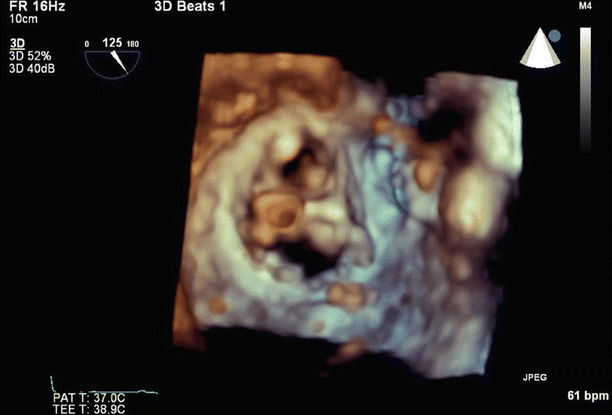
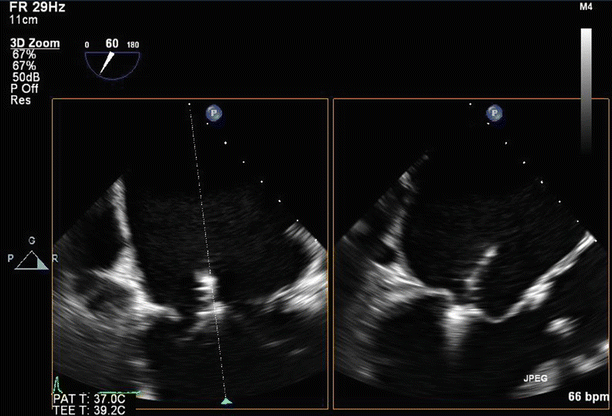
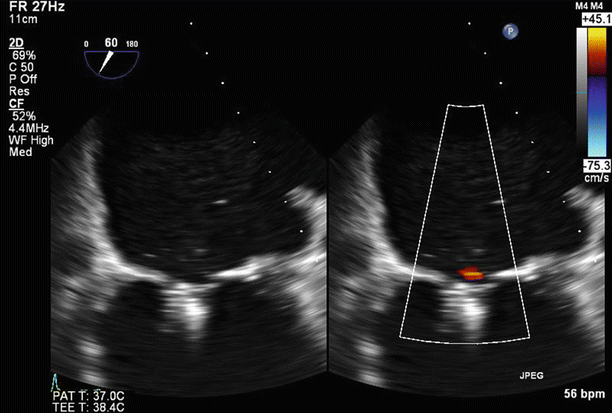
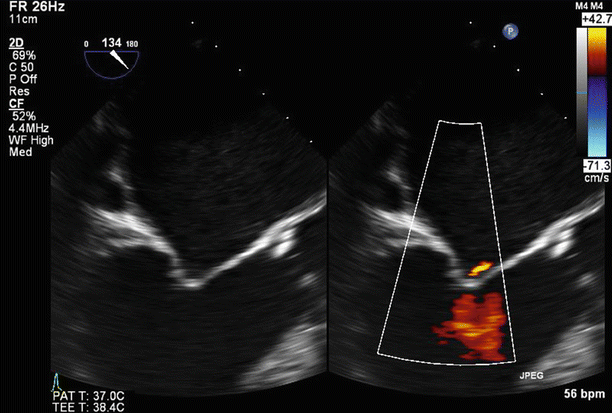
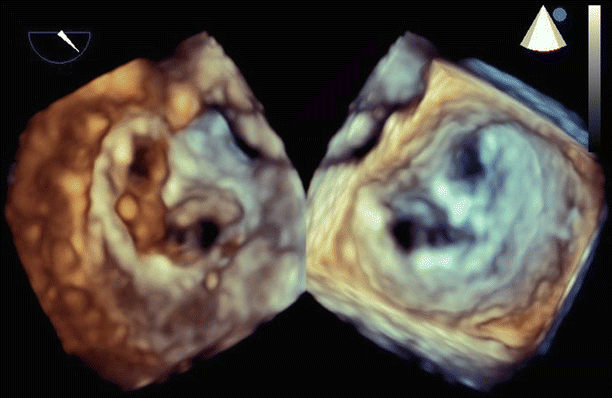
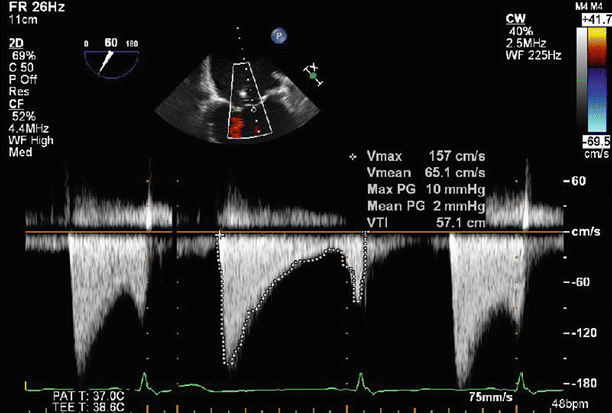
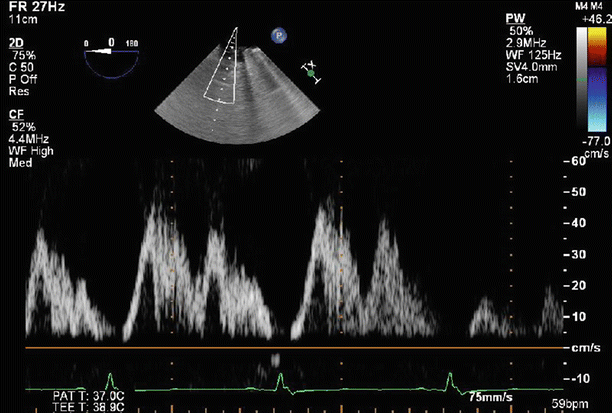
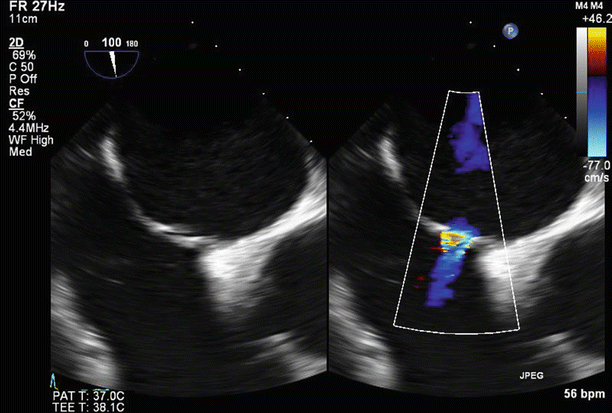

Fig. 6.6
Transesophageal echo (TEE) biplane view at 125° and 11°, demonstrating excessive motion and flail of the A2 segment of the anterior mitral valve leaflet

Fig. 6.7
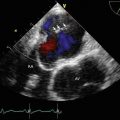
TEE biplane view at 125° and 11°, demonstrating severe posterolaterally directed mitral regurgitation (MR)

Fig. 6.8
A proximal isovelocity surface area (PISA) of 1.7 cm2 supports the diagnosis of severe MR

Fig. 6.9
Tenting of the interatrial septum to confirm the position prior to septostomy

Fig. 6.10
The guide catheter is then advanced across the interatrial septum to gain access to the left atrium

Fig. 6.11
The device is positioned in the left atrium to ensure appropriate medial-lateral and anterior-posterior alignment with the mitral valve

Fig. 6.12
The device can be seen approaching the valve, using 3D imaging

Fig. 6.13
3D imaging can be used to visualize the MitraClip crossing the mitral valve

Fig. 6.14
When both clip arms are adequately aligned, the clip is positioned perpendicular to the line of leaflet coaptation. Both leaflet tips must be fully inserted on both sides of the device between the grippers and clip arms to ensure stability of attachment

Fig. 6.15
After deployment on an initial MitraClip across the medial aspects of A2 and P2, there is good leaflet opposition with moderate (2+) residual MR

Fig. 6.16
3D zoom imaging is used to visualize the MitraClip from the left atrium. The clip is well positioned, with good attachment to both leaflets, and creates a double-orifice valve appearance

Fig. 6.17
The same double-orifice view can be appreciated in 3D from the left ventricular aspect of the mitral valve

Fig. 6.18
If there is residual MR, a second MitraClip is often deployed to improve leaflet coaptation. Care must be taken not to disrupt the first clip and to ensure that the valve orifice does not become stenotic. There is also increased risk of snaring the second clip in the subvalvular apparatus, as the chordae are now more centrally positioned after deployment of the first clip. In this example, deployment of a second clip is seen immediately adjacent to the first clip on the lateral aspect of the A2 and P2 scallops (biplane view)

Fig. 6.19
An excellent final result is achieved, with only trivial MR seen on this bicommissural view with simultaneous gray-scale and color Doppler images

Fig. 6.20
Trivial MR is confirmed on a long-axis view with simultaneous gray-scale and color Doppler images

Fig. 6.21
The adjacent MitraClips create a double-orifice mitral valve, which can be visualized in 3D from both the left ventricular (left image) and left atrial (right image) aspects

Fig. 6.22
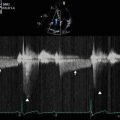
Continuous wave Doppler imaging across the mitral valve confirms that there is no hemodynamically significant stenosis related to deployment of the two MitraClips. Postprocedure transvalvular gradients were within normal limits (peak/mean 10/2 mmHg)

Fig. 6.23
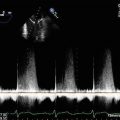
Pulse wave Doppler imaging of the left upper pulmonary vein profile demonstrates normalization of the spectral trace, suggesting that left atrial pressures have already been reduced, related to the significant reduction in MR severity

Fig. 6.24
An expected trivial to mild residual left-to-right interatrial shunt was noted at the septostomy site on color Doppler imaging
Video 6.1 Transesophageal echo (TEE) biplane view at 125° and 11°, demonstrating excessive motion and flail of the A2 segment of the anterior mitral valve leaflet (AVI 3872 kb)
Video 6.2 TEE biplane view at 125° and 11°, demonstrating severe posterolaterally directed mitral regurgitation (AVI 1180 kb)
Video 6.3 Tenting of the interatrial septum to confirm the position prior to septostomy (AVI 7690 kb)
Video 6.4 The guide catheter is then advanced across the interatrial septum to gain access to the left atrium (AVI 4432 kb)
Video 6.5 The device is positioned in the left atrium to ensure appropriate medial-lateral and anterior-posterior alignment with the mitral valve (AVI 6117 kb)
Video 6.6 The device can be seen approaching the valve, using 3D imaging (AVI 2332 kb)
Video 6.7 3D imaging can be used to visualize the MitraClip crossing the mitral valve (AVI 1962 kb)
Video 6.8 When both clip arms are adequately aligned, the clip is positioned perpendicular to the line of leaflet coaptation Both leaflet tips must be fully inserted on both sides of the device between the grippers and clip arms to ensure stability of attachment (AVI 4108 kb)
Video 6.9 After deployment on an initial MitraClip across the medial aspects of A2 and P2, there is good leaflet opposition with moderate (2+) residual MR (AVI 4226 kb)
Video 6.10 3D zoom imaging is used to visualize the MitraClip from the left atrium The clip is well positioned, with good attachment to both leaflets, and creates a double-orifice valve appearance (AVI 2359 kb)
Video 6.11 The same double-orifice view can be appreciated in 3D from the left ventricular aspect of the mitral valve (AVI 2311 kb)
Video 6.12 If there is residual MR, a second MitraClip is often deployed to improve leaflet coaptation Care must be taken not to disrupt the first clip and to ensure that the valve orifice does not become stenotic There is also increased risk of snaring the second clip in the subvalvular apparatus, as the chordae are now more centrally positioned after deployment of the first clip In this example, deployment of a second clip is seen immediately adjacent to the first clip on the lateral aspect of the A2 and P2 scallops (biplane view) (AVI 4300 kb)
Video 6.13 An excellent final result is achieved, with only trivial MR seen on this bicommissural view with simultaneous gray-scale and color Doppler images (AVI 5098 kb)
Video 6.14 Trivial MR is confirmed on a long-axis view with simultaneous gray-scale and color Doppler images (AVI 5190 kb)
Video 6.15 The adjacent MitraClips create a double orifice mitral valve, which can be visualized in 3D from both the left ventricular (left image) and left atrial (right image) aspects (AVI 2534 kb)
Video 6.16 An expected trivial to mild residual left-to-right interatrial shunt was noted at the septostomy site on color Doppler imaging (AVI 5364 kb)
6.3 Case 2. MitraClip for Posterior Leaflet Prolapse
An 87-year-old man presented with progressive dyspnea on minimal exertion. He had known severe (4+) mitral regurgitation due to posterior leaflet prolapse with P2 flail and moderately severe pulmonary hypertension (right ventricular systolic pressure [RVSP] 73 mmHg). He declined surgery but was offered percutaneous mitral valve repair with a MitraClip. After the intervention, there was improvement in mitral regurgitation to 1+ and no associated stenosis (mean gradient of 2 mmHg) (Figs. 6.25, 6.26, 6.27, 6.28, 6.29, and 6.30).
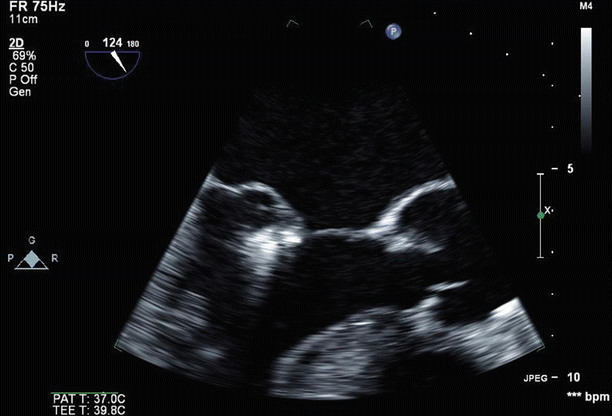
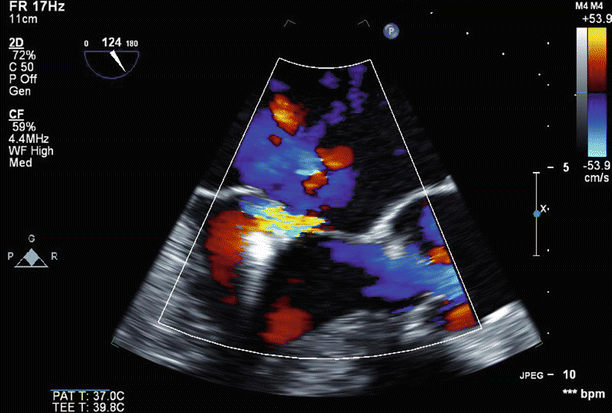
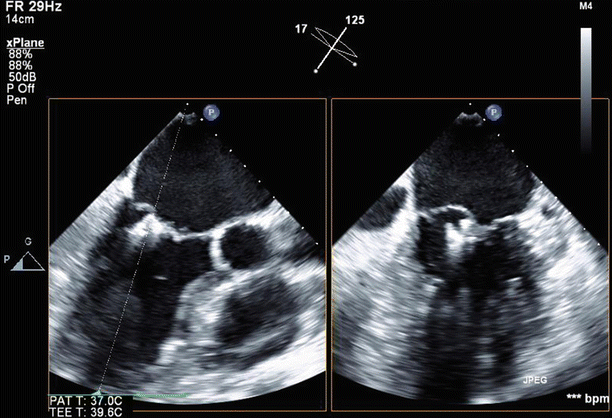
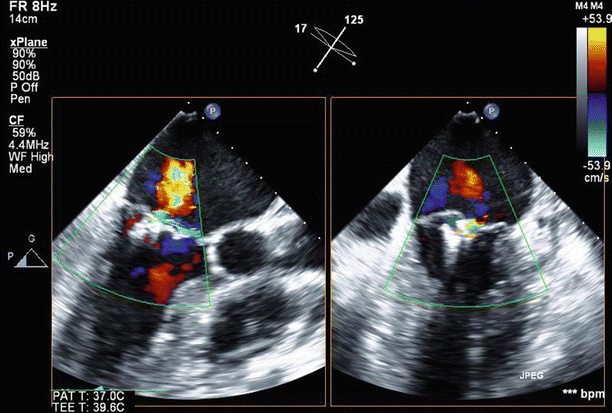
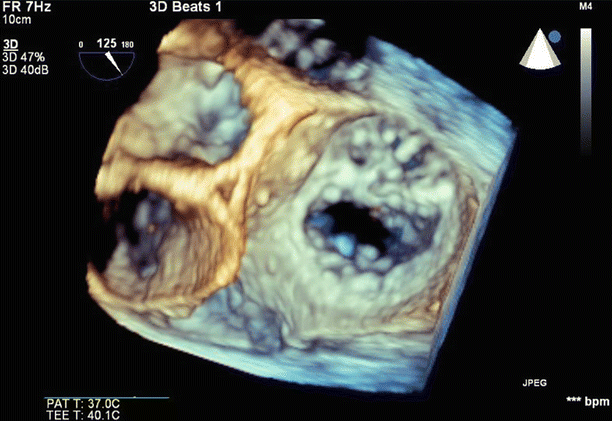
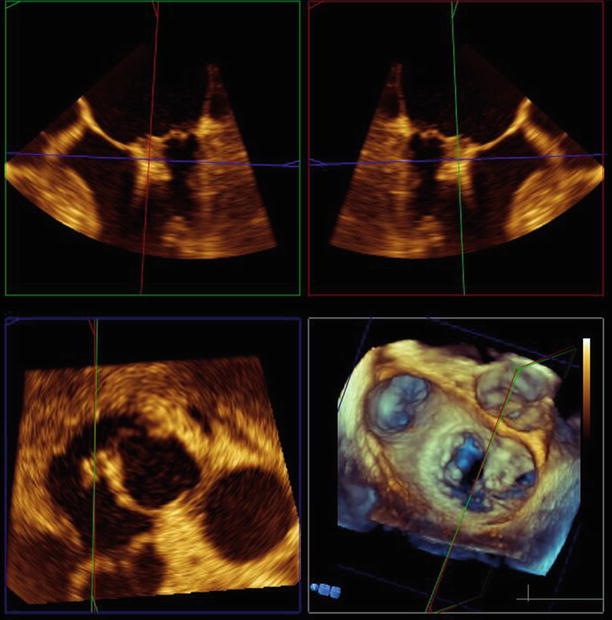

Fig. 6.25
TEE 120° view shows the mitral valve with two MitraClips deployed side-by-side on the central and medial aspects of the valve, with good leaflet coaptation

Fig. 6.26
TEE 120° view demonstrates mild (1+) residual central MR

Fig. 6.27
Biplane view of the mitral valve in long-axis (125°) and off-axis four-chamber views (17°), again showing the MitraClips

Fig. 6.28
Biplane view of the mitral valve in long-axis (125°) and off-axis 4 chamber views (17°) with the MitraClips in situ and mild (1+) residual MR

Fig. 6.29
3D zoom view of the mitral valve from the left atrial perspective. The Mitraclips can be seen side-by-side, apposing the anterior and posterior leaflets

Fig. 6.30
Use of Philips 3DQ reconstruction software demonstrates a multiplanar view of the mitral valve with the MitraClips in situ
Video 6.17 TEE 120° view shows the mitral valve with two MitraClips deployed side-by-side on the central and medial aspects of the valve, with good leaflet coaptation (AVI 10853 kb)
Video 6.18 TEE 120° view demonstrates mild (1+) residual central MR (AVI 2617 kb)
Video 6.19 Biplane view of the mitral valve in long-axis (125°) and off-axis (17°) four-chamber views, again showing the MitraClips (AVI 5001 kb)
Video 6.20 Biplane view of the mitral valve in long-axis (125°) and off-axis 4 chamber views (17°) with the MitraClips in situ and mild (1+) residual MR (AVI 1003 kb)
Video 6.21 3D zoom view of the mitral valve from the left atrial perspective The Mitraclips can be seen side-by-side, apposing the anterior and posterior leaflets (AVI 1076 kb)
Video 6.22 Use of Philips 3DQ reconstruction software demonstrates a multiplanar view of the mitral valve with the MitraClips in situ (AVI 1554 kb)
6.4 Case 3. MitraClip for Anterior Leaflet Prolapse
An 89-year-old man with a history of previous aortic valve replacement and known anterior mitral prolapse presents with increasing dyspnea (including paroxysmal nocturnal dyspnea), peripheral edema, and palpitations. He was noted to have an estimated RVSP of 95 mmHg, consistent with severe pulmonary hypertension. He was felt to be a very high-risk candidate for redoing open surgery, considering his age and problems with the previous surgery, so he was offered the option of MitraClip instead of open surgery (Figs. 6.31, 6.32, 6.33, 6.34, 6.35, 6.36, 6.37, 6.38, 6.39, 6.40, 6.41, and 6.42).
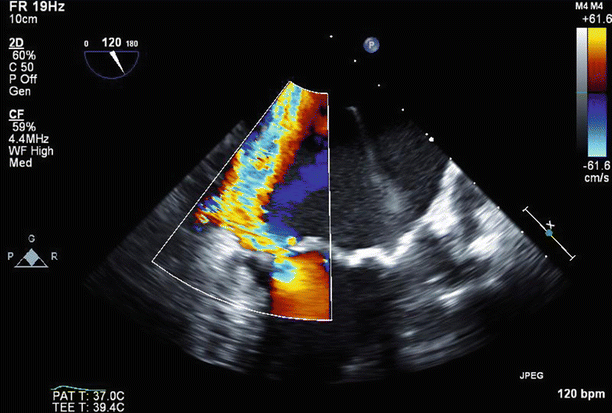
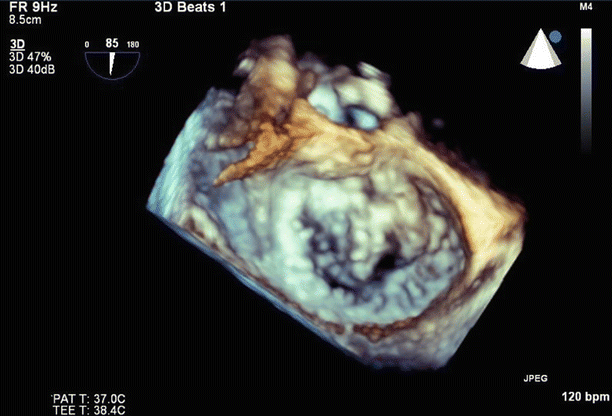
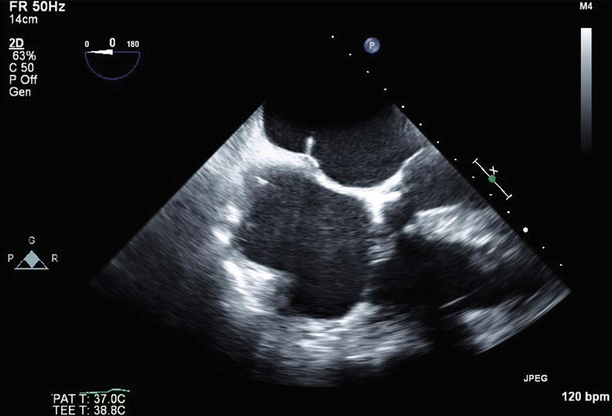
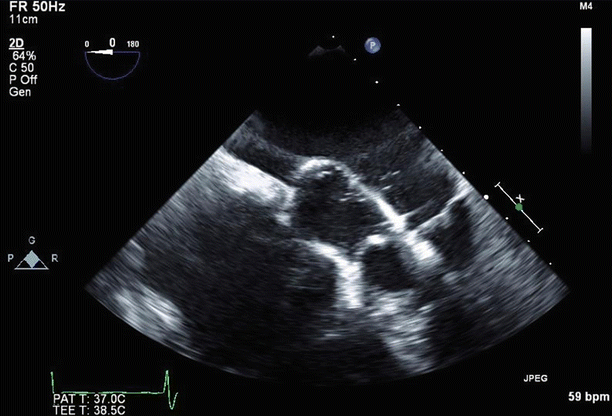
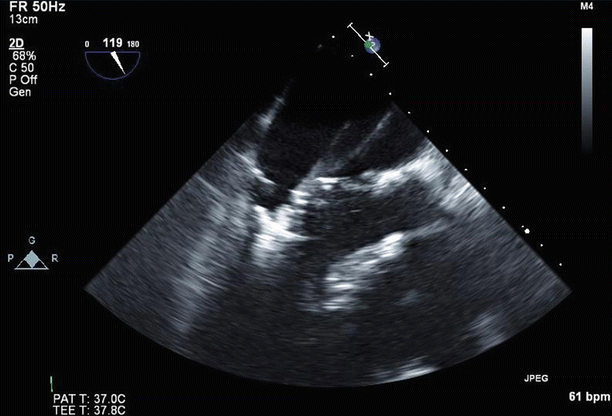

Fig. 6.31
Color Doppler imaging in a long-axis (120°) view confirmed severe (4+), posteriorly directed mitral regurgitation due to anterior leaflet prolapse and partial flail

Fig. 6.32
3D imaging of the mitral valve from the left atrium confirmed predominant involvement of the A1/A2 scallops

Fig. 6.33
Atrial septostomy was performed

Fig. 6.34
The device is aligned with the mitral valve using the steerable guide catheter, which bends at 90° in the left atrium after traversing the interatrial septum

Fig. 6.35




The device is aligned perpendicular to the valve leaflets. The long-axis (120°) view is helpful to visualize both device arms and both mitral valve leaflets simultaneously
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree