Substrate
Invasiveness
Clinical decision making
Urine
−
Biopsy
Blood
−
Biopsy
Biopsy specimen
+
Treatment
Prostatectomy specimen (Gleason score and pTNM)
++
Adjuvant treatment
PSA
Total PSA
In 1986, PSA was approved by the Food and Drug Administration as a marker to monitor treatment in patients with prostate cancer, and in 1994 as a diagnostic marker. It is currently the only widely used marker for prostate cancer.
PSA, also known as kallikrein 3 or hK3, is a serine protease that is a member of the family of glandular kallikrein-related peptidases. The genes for the glandular kallikreins are clustered at chromosome 19q133-4 and transcription of PSA is regulated by androgens [7]. The function of PSA is to liquefy seminal fluid through its action on the gel-forming proteins semenogelin and fibronectin [8].
PSA is not a cancer-specific marker, as it is produced by both benign and malign prostate epithelial cells. Normally, PSA blood levels are low. A healthy prostate is surrounded by a continuous layer of basal cells and a basement membrane which prevent the high concentrations of PSA in the prostate to leak into blood. High PSA blood levels can be caused by an elevated synthesis or an increased release of PSA into blood. An elevated PSA synthesis can be a result of benign prostatic hypertrophy (BPH) and prostate manipulation [9, 10]. PSA expression, ergo PSA synthesis, is slightly decreased in the development and progression of prostate cancer [11]. Therefore, as is seen in prostatitis, the increased PSA blood levels in prostate cancer are assumed to be a result of an increased release of PSA into blood through the disrupted architecture of the prostate.
Despite extensive research, difficulty persists in defining the optimal cutoff value for PSA. Traditionally, it was set at 4.0 ng/ml. Using this PSA cutoff provides a sensitive test, with a positive predictive value of 37% and a negative predictive value of 91% [12]. In other words, 75% of men with PSA 4.0–10.0 ng/ml who undergo biopsy do not have cancer [13]. In addition, several studies showed a substantial probability of prostate cancer within the PSA interval 0–4.0 ng/ml [14–16]. The Prostate Cancer Prevention Trial (PCPT), for example, reported that 27% of men with normal DRE and a serum total PSA between 3.1 and 4.0 ng/ml have prostate cancer [16]. On the other hand, it has never been demonstrated that lowering the PSA cutoff affects the long-term survival in men with prostate cancer. Furthermore, this will most likely lead to a higher number of unnecessary biopsies and an increased detection of clinical insignificant prostate cancer. Other factors of influence on PSA blood level are ethnic background and the use of medication. Men from African descent have higher PSA levels than Caucasian men, even after adjusting for prostate volume [17, 18]. And men using 5α-reductase inhibitors for treatment of BPH (such as dutasteride and finasteride) will have lower PSA levels by an average of 50% after 6 months of treatment [19, 20].
Several studies report that PSA measured before age 50 might be indicative for the risk of developing prostate cancer years or even decades later [21, 22]. It is also suggested that total PSA level at age 44–50 might also predict the likelihood of developing advanced prostate cancer, defined as clinical T3 or higher or metastatic disease at time of diagnosis [23]. This, however, needs further validation before possible implementation into clinical practice.
Risk Calculators
Risk calculators including several predictive factors to stratify patients for prostate biopsy have been developed. Two well-known calculators that are available online are the PCPT and the ERSPC risk calculator [24, 25]. The first includes serum PSA, DRE results, age, family history of prostate cancer, ethnicity, and prior biopsy. The latter includes serum PSA, DRE results, TRUS findings, prior biopsy, and prostate volume. The use of risk calculators allows a more individual assessment of prostate cancer risk and provides a better predictive accuracy compared to PSA alone [26].
PSA Derivatives
PSA derivates have been evaluated in the attempt to enhance the diagnostic accuracy of total PSA: age-specific total PSA cutoffs, total PSA density, total PSA velocity, and total PSA doubling time. Age-specific PSA cutoff values were suggested to enhance the predictive value of PSA. The suggested cutoff values were 40–49 years old: 2.5 ng/ml, 50–59: 3.5 ng/ml, 60–69: 4.5 ng/ml, and 70–79: 6.5 ng/ml. However, the use of an age-specific total PSA cutoff is not validated and criticized for missing clinically significant cancers in older men [27].
PSA density is defined as the total serum PSA level divided by the volume of the prostate (in grams). A PSA density of 0.15 ng/nl/g or higher has been considered abnormal and suspicious for cancer. However, the value of this test remains controversial [28]. PSA density correlated with biopsy outcome, tumor aggressiveness, and unfavorable pathological features in several studies [29–31]. However, other studies could not validate these results [32, 33]. In addition, PSA density requires transrectal ultrasound, which is time-consuming, expensive, and causes patient discomfort. All together, PSA density is not widely used in clinical practice.
PSA dynamics have been extensively studied for their assumed predictive value to discriminate between benign and malign conditions of the prostate. This includes PSA velocity, the change in PSA over time, and PSA doubling time, the number of months for a certain level of PSA to increase by a factor of two. PSA dynamics are indisputably correlated with the diagnosis of prostate cancer on biopsy. However, there is no sufficient evidence that PSA velocity or PSA doubling time has additional diagnostic value beyond the use of total PSA. Thus, there is no justification for the use of PSA dynamics in clinical decision making before treatment in early stage prostate cancer [34]. PSA dynamics are however valuable to monitor treatment. Recurrence after radical prostatectomy can be monitored with high sensitivity using PSA doubling time. Although currently widely used, PSA response to chemotherapy in castrate-resistant prostate cancer patients does not predict long-term benefit adequately.
PSA Molecular Forms
PSA circulates in blood either in a stable complexed form or in an unbound “free” form. Complexed PSA is bound to proteins: α1-antichymotrypsin (ACT), α2-macroglobulin (A2M), and α1-protease inhibitor (API). A lower percent free PSA (free PSA/total PSA × 100) is correlated with a higher probability of finding prostate cancer on biopsy [35, 36]. The use of percent free PSA has been approved as a diagnostic marker by the Food and Drug Administration in men with PSA levels 4.0–10.0 ng/ml. A cutoff value of 25% is generally used. Note that free PSA is less stable than complexed PSA, causing greater analytic variability. Suboptimal blood sample handling can considerably influence free PSA levels [37].
Free PSA exists in different molecular isoforms, including pro-PSA, BPH-associated BPSA, and intact free PSA [38, 39]. Several studies report significantly higher levels of pro-PSA in patients with prostate cancer, and decreased levels of BPSA and intact free PSA [40–42]. This implies that pro-PSA might be a purer biomarker for prostate cancer than free-PSA. Pro-PSA has also been suggested to selectively identify patients with more aggressive prostate cancer. Its suggested additional diagnostic and prognostic value has yet to be validated.
Novel Prognostic Biomarkers
Tissue Markers
Once tissue is available, important decisions have already been made, either a biopsy has been taken or the gland was surgically removed. Thus, the main clinical need is to accurately predict the biological behavior of the malignant process. In case the pathologist is not sure about the diagnosis of invasive prostate cancer, immunohistochemistry using antibodies against the basal cell-specific high molecular weight keratins (34β E12) and AMACR has proven to be helpful [43]. It is striking that this is the only molecular pathological application that has been widely accepted and used in prostate cancer. Numerous studies report on the potential of biomarkers detected by immunohistochemistry, yet none is routinely used for a better assessment of prognosis. Whereas for other malignancies biomarkers that predict progression of the disease in patients who were treated with curative intend are routinely used (e.g., breast and colon cancer), so far there has not been a great interest in adjuvant treatment of patients with high-risk localized prostate cancer. The most significant study in this respect was the EPC initiative, in which a stratification on standard clinical and pathological risk factors was used. Now better treatment modalities become available, adjuvant strategies are likely to be considered again, and biomarkers indicative for biological behavior, determined in tissue, will be needed. In this part, we will focus on high potential biomarkers for which standardized methods are or can be developed.
Gene Fusions: TMPRSS2-ERG
The classic example of a gene fusion implicated in cancer development is the BCR:ABL fusion in patients with chronic myelogenous leukemia. This fusion results from a reciprocal translocation T(9;22), first recognized as the Philadelphia chromosome. This discovery has been revolutionary as it has led to the development of imatinib [44]. This is an inhibitor of the BCR: ABL gene fusion product which transformed the previously fatal leukemia into a manageable chronic disease for many patients.
In prostate cancer, recurrent gene rearrangements were discovered in 2005: a fusion of the 5′ untranslated region of TMPRSS2 (androgen-regulated trans-membrane protease, serine 2) to Ets family genes (oncogenic transcription factors) [45]. Oncogene ERG (v-ets erythroblastosis virus E26 oncogene homolog (avian)) is the most commonly involved Ets family member in gene fusion. TMPRSS2–ERG has been detected in approximately 50% of Caucasian prostate cancer patients. This gene fusion is less frequently seen in men from other ethnic background. A recent study reported fusion positive prostate cancers in 31% of African–American men and only in 16% of Japanese men [46]. Rearrangements with other Ets transciption factors have been identified in approximately 5–10% of PSA screened prostate cancers: ETS variant 1 gene (ETV1), ETV4, and ETV5 [47–49]. In addition to TMPRSS2, other fusion partners involved in ETS fusions have been identified. Their possible clinical relevance is not clear.
As a result of gene fusion with TMPRSS2, the expression of ERG becomes androgen regulated and thus overexpressed. ERG expression can be detected in prostate cancer patients by immunohistochemistry with a high specificity of >95% and is not seen in benign prostate epithelium [50, 51]. This suggests that ERG immunostaining could be a solid diagnostic biomarker, albeit in approximately half of the prostate cancer patients. The clinical relevance of Ets gene fusions is currently under investigation. Results on a potential prognostic value are conflicting. A worse prognosis of fusion positive cancers has been reported by several studies [52–54]. Other studies could not validate these results [55, 56] or found a favorable prognostic association [57, 58]. A recent large study showed that ERG status had no influence on the risk of PSA recurrence after radical prostatectomy [51]. In addition, they report a strong association between ERG positivity and high androgen receptor expression levels. This suggests that ERG status might have predictive value for response to antiandrogen therapy. However, this requires further investigation, before implementation into clinical practice can be realized.
Ki-67/MIB1 Labeling Index
Expression of the Ki-67 protein is strictly associated with cell proliferation. Ki-67 has therefore been extensively studied for its potential use as a proliferation marker in different types of cancer, including prostate cancer. Its name is derived from the city of origin (Kiel) and the number of the original clone in the 96-well plate [59]. Ki-67 can be determined by immunohistochemistry using the monoclonal antibody MIB-1 [60]. The proportion of tumor cells staining positive for Ki-67 is known as the Ki-67 labeling index. This proved to be an independent and significant prognostic biomarker for prostate cancer-specific survival [61, 62]. Furthermore, the Ki-67 labeling index has been repeatedly shown to be a predictive marker for disease recurrence and progression after radical prostatectomy and radiotherapy [63–65]. Although its usefulness has been well established, the Ki-67 labeling index is currently not used in daily practice.
Phosphatase and TENsin Homolog
Phosphatase and TENsin homolog (PTEN) is a tumor-suppressor gene, located on chromosome 10q23 [66]. This gene plays a key role in carcinogenesis. PTEN antagonizes the PI-3K/Akt pathway and thereby modulating cell growth/survival and cell migration/adhesion [67]. In prostate cancer, PTEN loss has been associated with proliferation and survival of cancer cells, resistance to castration [68], chemotherapy [69, 70] and radiotherapy [71], bone metastasis [72], and recurrence after radical prostatectomy [73]. Thus, PTEN is assumed to be a potent prognostic marker and a clear target for novel (gene) therapies. However, this requires further research.
E-Cadherin
Cadherins are a family of epithelial cell–cell adhesion molecules that play a key role in preserving epithelial integrity [74]. Their function is dependent on calcium, hence their name “calcium-dependent adhesion”. E-cadherin is the most extensively studied member of the cadherin family. During cancer progression to an invasive state, intercellular adhesions between tumor cells are disrupted. Thus, aggressive tumor cells were hypothesized to have loss of E-cadherin. And indeed, decreased E-cadherin expression has repeatedly been shown to correlate with a loss of tumor differentiation and a poor prognosis [75–77]. This correlation has been shown for several tumor types, including prostate cancer. However, large prospective studies will have to define its potential clinical relevance in prostate cancer, as a prognostic biomarker or as a molecular target for therapy.
EZH2
The EZH2 gene (Enhancer of zeste homolog 2), encoding a Polycomb-group (PcG) protein, is responsible for maintaining the silent state of genes. EZH2 mediates trimethylation of histone H3 lysine 27 (H3K27), leading to repression of transcription and thereby silencing of gene expression [78, 79]. EZH2 is upregulated in various aggressive tumors, including prostate cancer [80–82]. Furthermore, it mediates transcriptional silencing of the tumor suppressor gene E-cadherin [83]. This demonstrates an inverse correlation between dysregulation of EZH2 and repression of E-cadherin during cancer progression. In conclusion, EZH2 upregulation might play a key role in oncogenesis and progression of cancer. This makes it a promising biomarker of disease progression and a viable target for therapeutic interventions in aggressive cancers.
The Neuroendocrine Phenotype
The expression of a neuroendocrine phenotype in prostate cancer has been reported almost 25 years ago [84]. There is in good agreement that the relative fraction of cells with a NE phenotype increases in advanced prostate cancer, yet the use to predict biological behavior in localized prostate cancer remains controversial. Only in case of a “pure” NE phenotype, in small cell prostate cancer, a rare entity (<1% of all prostate cancer), the biology of the disease is markedly different from adenocarcinoma of the prostate, and therefore, treatment of this type of prostate cancer is different.
In summary, we can conclude that a robust set of candidate prognostic biomarkers is available that can be measured by immunohistochemistry. Stratification of patients based on these markers is well within reach, provided the methods and scoring systems are standardized.
Blood Markers
MicroRNAs
The discovery of microRNAs (miRNA) in 2004 was a revolutionary step in understanding the mechanisms regulating gene expression and function [85, 86]. Subsequently, it was reported that miRNAs play an important role in cancer by initiating carcinogenesis and driving progression [87].
MiRNAs are small endogenous noncoding RNAs, up to 22 nucleotides long, that regulate gene expression posttranscriptionally. MiRNAs bind to complementary sequences within messenger RNAs (mRNA) to alter their translation by inhibiting their translation or inducing the cleavage of specific target mRNAs [88]. In most cases, miRNAs “fine-tune” protein expression (only a modest reduction of the target mRNA concentration) [89]. Occasionally, it causes upregulation or complete destruction of the target mRNA [89–91].
MiRNAs are known to regulate common cellular targeted pathways (intracellular signaling, DNA repair, and cellular adhesion/migration) [92–94], androgen signaling [95–97], and apoptosis avoidance [98, 99]. The exact role of miRNAs in the development and progression of prostate cancer is still being investigated. Yet, miRNAs are promising potential biomarkers and novel therapeutic targets for prostate cancer.
Circulating Tumor Cells
The importance of circulating tumor cells (CTC) was already acknowledged in 1869 by Thomas Ashworth, an Australian physician who observed CTCs microscopically [100]. Only recent advances in technology facilitate a reliable method for the detection of CTC in blood. The presence of CTCs in blood proved to be associated with overall survival in patients with metastatic breast [101, 102], colorectal [103, 104], and prostate cancer [105, 106].
In castrate-resistant prostate cancer (CRPC), CTC number before and after treatment is an independent predictor of survival. This is a strong predictor both as a continuous variable and when using discrete cutoff values (≥5 CTC/7.5 ml of blood vs. <5 CTC) [105–107]. Posttreatment CTC number showed to be a stronger prognostic factor for survival than a 50% decline in PSA (AUC 0.87 vs. 0.62). CTCs are approved by the Food and Drug Administration as a prognostic biomarker to monitor disease status in patients with metastatic breast, colorectal, and prostate cancer. To further explore the potential link to survival, CTCs have been incorporated as an exploratory endpoint in several phase II and III trials [108].
hK2 and uPA
Human kallikrein 2 (hK2) and urokinase plasminogen activation (uPA) are potential future prostate cancer biomarkers that are thus far not validated. HK2 is from the same gene family as PSA, but differs in its enzymatic activity [109]. Several studies have shown that the use of a combination of hK2 with free and total PSA might improve the predictive value for prostate cancer [110, 111]. HK2 might also have prognostic value [112, 113]. The serum protease uPA might be involved in tumor development and progression through degradation of the extracellular matrix [114]. The potential role of uPA as a biomarker of metastatic prostate cancer needs to be validated in large multicenter studies.
Urine Markers
PCA3
In 1999, Bussemakers et al. first identified and characterized the differential display clone 3 (DD3, later called PCA3) gene, to date one of the most prostate cancer specific genes [115]. PCA3 is noncoding RNA and located on chromosome 9q21–22. Its function is unknown. PCA3 is highly overexpressed in prostate tumors compared to adjacent benign prostate tissues, on average between 70- and 80-fold. An upregulation is seen in 95% of the primary prostate tumors and no PCA3 expression is found in non-prostate tissue (i.e., benign and malign tissue from breast, cervix, endometrium, ovary, and testis; cell lines originating from bladder, kidney, and ovarian cancer) [115].
In the initial PCA3 studies, a real-time RT-PCR analysis was used for the quantification of PCA3 messenger RNA (mRNA) in prostate tissue. Later, Hessels et al. developed a dual time resolved fluorescence (TRF)-based RT-PCR assay to detect PCA3 mRNA in urinary sediments after DRE [116]. A urine test provides a noninvasive method to obtain prostate (cancer) cells, which makes it suitable for clinical purposes. A DRE is performed to mobilize prostatic cells towards the prostatic urethra, which are flushed out with the first voided urine. A prostate massage is obsolete and causes needless patient discomfort, as a regular DRE sheds enough cells into urine for analysis. In 2006, the Progensa PCA3 test was introduced, a transcription-mediated amplification (TMA) assay [117]. This assay is also performed on first voided urine samples after DRE, but it is a simpler, faster, and sensitive enough method compared to the initial RT-PCR based assay; therefore, it is more viable for widespread clinical implementation. The PCA3 score is the ratio of PCA3:PSA mRNAs multiplied by 1,000. The Progensa PCA3 test is commercially available and Conformité européenne (CE)-approved since November 2006 to aid in the decision to take initial or repeat biopsies. The Food and Drug Administration approval process is currently ongoing.
The clinical utility of PCA3 and its additional predictive value beyond PSA has been extensively studied. PCA3 has been validated as a reliable predictor of prostate cancer at initial or repeat biopsy [116, 118–121]. Currently, a cutoff value of 35 is used, resulting in a sensitivity of 47–69% and a specificity of 72–79% [117, 119–121]. However, the optimal cutoff value is subject to debate. Several studies indicate that a cutoff value of 20 or 25 might be preferable, missing less prostate cancers and still preventing a considerable amount of prostate biopsies [118]. Future studies will have to clarify this issue. Furthermore, PCA3 showed to be an independent predictor of prostate cancer in addition to established prostate cancer risk factors (age, PSA, DRE, prostate volume, and biopsy history) [122, 123]. The use of PCA3-based nomograms has recently been validated [124], providing a novel tool for clinical decision making.
It was hypothesized that PCA3 might be associated with more aggressive cancer. This was based on the theory that aggressive prostate cancer cells are more invasive and would therefore more easily shed into the prostatic ductal system after DRE [125]. However, to date, the prognostic value of PCA3 is considered to be limited. Some studies found a correlation of PCA3 with Gleason score [118, 120, 126], but this is contradicted by a range of other studies that show no (additional) predictive value for Gleason score [125, 127–129]. As concluded by Auprich et al., the clinical value of PCA3 to predict aggressive prostate cancer at radical prostatectomy seems to be marginal at best [127]. PCA3 has been shown, however, as a valuable predictor of tumor volume and insignificance of prostate cancer [120, 127, 129]. Data on predictive value for extracapsular extension are conflicting [120, 127, 130]. Furthermore, PCA3 currently has no role in risk assessment during active surveillance protocols, though this requires further investigation in larger studies [129, 131].
Gene Fusions: TMPRSS2–ERG
For a complete description of the gene fusion TMPRSS2-ERG, see section tissue markers. In summary, TMPRSS2–ERG is a fusion of TMPRSS2 (the androgen-regulated trans-membrane protease, serine 2) to Ets family genes (oncogenic transcription factors). Oncogene ERG is the most commonly involved Ets family member in gene fusion. It occurs in approximately half of Caucasian prostate cancer patients.
A publication in 2006 showed the feasibility to detect TMPRSS2–ERG fusion transcripts noninvasively in urinary sediments obtained after DRE using an RT-PCR-based research assay [132]. Since then, extensive research has been performed on the clinical applicability of this urine test. A sensitivity of 37% and specificity of 93% to predict prostate cancer were reported, resulting in a positive predictive value of 94% [133]. Although not (yet) validated, this test is assumed to improve the specificity of established prostate cancer risk calculators.
Urine Marker Panel
Given the tumor heterogeneity in prostate cancer, the use of a panel of biomarkers may provide the best diagnostic accuracy. Hessels et al. evaluated the combination of PCA3 with TMPRSS2–ERG fusion transcripts detected in the urine, showing an improved sensitivity of 73%, compared to 62% for PCA3 alone, without compromising the specificity for detecting prostate cancer [133]. A recent study confirmed an enhanced predictive value of PCA3 combined with TMPRSS2–ERG [134]. In conclusion, these preliminary results on the combined use of PCA3 and TMPRSS2-ERG seem promising but require further validation. Future studies will have to assess the use of other (novel) biomarker panels.
Future Perspectives
In the worldwide search for novel prognostic biomarkers for prostate cancer, many tumor markers have been proposed. The number of articles published on this subject has increased substantially in the last decade. However, PSA, PCA3, and CTCs are still the only ones used in clinical practice. Many published results on novel prostate cancer biomarkers appear not reproducible in subsequent studies and thus will never attain the FDA approved status (Table 6.2). Where a double-blind randomized placebo-controlled trial is the gold standard for therapeutic studies, biomarker studies are not regulated by clear guidelines. These studies often suffer poor study design, lack methodological quality, and standardized assays, and information on key elements of design and analysis are often not reported. To improve the quality of diagnostic studies, the STAndards for Reporting of Diagnostic accuracy (STARD) statement was developed by a group of scientists and editors in 2003 [135]. It consists of a checklist of 25 items and flow diagram that authors can use to ensure that all relevant information is present. In addition, the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines were published in 2005 [136]. These are guidelines for transparent and complete reporting of studies so that poor studies can be better identified. These initiatives are important steps forward in improving the quality of tumor marker studies, but further improvement of future studies is warranted.
Table 6.2
The different stadia of biomarker research
Stadia of biomarker research | Corresponding markers in prostate cancer |
|---|---|
1. Exploratory, no intended use cohort | microRNA, uPA, EPCA-1, EPCA-2, etc. |
2. Research use only assay, evaluated retrospectively | hK2, PTEN, Ki-67, EZH2, E-Cadherin |
3. Research use only assay, evaluated prospectively | TMPRSS2–ERG |
4. CE/FDA approved | PSA, PCA3, circulating tumor cells |
Other future improvement includes the use of a secured database with audit-trail so that results cannot be manipulated after analysis. Validation of a potential novel biomarker should only be approved after multiple prospective studies with an “intended use” cohort. Furthermore, it should be kept in mind that it is not sufficient to show that a potential novel biomarker is statistically significant in multivariate analysis: it should improve the predictive accuracy of the multivariate model. In conclusion, future biomarker studies should meet the STARD criteria and should be reported in compliance with the REMARK guidelines.
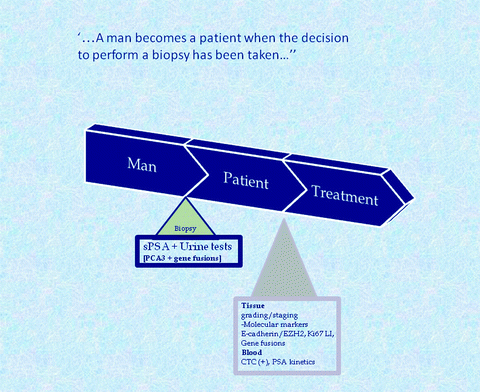
So, many new biomarkers are ready for “prime time,” yet it needs carefully designed studies to test the exact clinical positioning. In the clinical arena, two main themes can be discriminated (Fig. 6.1). Develop methods to better predict biopsy outcome; once the decision to take a biopsy has been taken, the man is a patient, a patient with or without prostate cancer. This is a tough challenge since the man with indolent cancer should not be bothered with a biopsy, yet the ones in the low PSA ranges with aggressive disease should be identified. Once the cancer is diagnosed, we should better predict the prognosis and therapy need/response. This will require significant efforts from molecular pathology.