Chapter Outline
World Health Organization and RECIST 1.0 Guidelines
Other Tumor Response Assessment Criteria
Functional Magnetic Resonance Imaging Techniques
Imaging plays a crucial role in the objective assessment of tumor response to various cancer therapies. Assessment of tumor response serves as an important clinical endpoint in phase II clinical trials and can reasonably predict the overall survival and other clinical events, such as time to progression. More than 70% of recent Food and Drug Administration approvals of oncologic drugs have been based on radiologic response. Several response assessment criteria have been used in oncology for assessment of response to gastrointestinal malignant neoplasms. Not only are these for use in research or drug trials, but they may also be incorporated into routine clinical practice. Early detection of therapy resistance may enable individualized treatment approaches tailored to the patient.
World Health Organization and RECIST 1.0 Guidelines
The World Health Organization (WHO) tumor response criteria, published in 1981, recommended the use of the cross product obtained by multiplying the longest diameter in the axial plane and the largest perpendicular diameter. Treatment response was categorized as complete response, indicating tumor disappearance; partial response, indicating more than 50% decrease in cross product; disease progression, indicating more than 25% increase in cross product; or stable disease, indicating changes that lay between partial response and disease progression. The WHO criteria suggested that in the presence of multiple lesions, the sum of the cross products of individual target lesions may be used to categorize the response. Since their inception, the WHO criteria were widely used for more than two decades. However, their deficiencies became obvious over time. No indication was given of the minimum number of lesions or the minimum size for a lesion to be categorized as a target lesion, and there were no guidelines on what type of imaging modality may be used. The bidimensional measurements were cumbersome, with minor errors in measurement resulting in a large change to the cross product. To alleviate these difficulties, several modifications were attempted, but none of these methods was uniformly accepted.
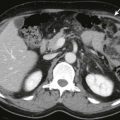
In the year 2000, the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines were published by a task force that included members of the European Organization for Research and Treatment in Oncology, the National Cancer Institute of the United States, and the National Cancer Institute of Canada. This recommendation was based on a retrospective evaluation of more than 4000 patients in 14 different trials. These guidelines addressed most but not all of the deficiencies in the WHO criteria. RECIST guidelines recommended the use of one-dimensional tumor measurements (the longest diameter) rather than the cross product ( Fig. 127-1 ). Lesions were categorized as measurable and nonmeasurable; those measuring more than 20 mm on conventional imaging techniques or more than 10 mm on helical computed tomography (CT) were considered measurable. The measurable lesions were categorized as target and nontarget lesions on the basis of the size and reproducibility of measurements. Only target lesions were used for response categorization. The RECIST guidelines recommended that up to 10 target lesions, in up to five organs, may be used in response assessment. The nontarget lesions were not measured unless there was an unequivocal progression of these lesions that indicated progressive disease. As in the WHO criteria, the tumor responses were complete response, indicating the disappearance of all target lesions; partial response, indicating a decrease of more than 30% in the sum of the greatest dimension of target lesions; progressive disease, indicating an increase of more than 20% in the sum of the greatest dimension of target lesions or the appearance of new lesions or unequivocal progression of nontarget lesions; and stable disease as tumors not classified in the other three classes. Major differences between the WHO and RECIST criteria are highlighted in Table 127-1 .

| WHO | RECIST 1.0 | |
|---|---|---|
| Imaging modality | No particular mention of imaging modality | CT, MRI, and chest radiography are recommended modalities |
| Definition of measurable lesions | Should be measurable in two dimensions; no limitation on minimal size of the lesion | Should be accurately measurable in at least one dimension; longest diameter >20 mm for nonspiral CT and >10 mm for spiral CT |
| Method of measurement | Cross product of longest diameter and greatest perpendicular diameter | Longest diameter in axial plane |
| Number of lesions to be measured | No particular number of lesions specified | Up to total of 10 target lesions (5 per organ) measured |
| Response evaluation |
|
|
Despite the several improvements over WHO guidelines, RECIST 1.0 guidelines did not address many relevant issues, including the measurement of lymph nodes, the utility of newer functional imaging techniques such as positron emission tomography with 18 F-fluorodeoxyglucose ( 18 F-FDG PET) and perfusion magnetic resonance imaging (MRI), and the assessment in clinical trials using noncytotoxic drugs.
Evolution of RECIST 1.1
In an effort to address these limitations, the RECIST working group made several modifications to the existing criteria after evaluation of prospectively documented data from clinical trials with a total of more than 6500 patients who had more than 18,000 target lesions. Major differences between RECIST 1.1 and RECIST 1.0 are highlighted in Table 127-2 .
| RECIST 1.0 | RECIST 1.1 | |
|---|---|---|
| LESION MEASUREMENT | ||
| Minimum size of measurable lesions | CT: 10 mm, spiral; 20 mm, nonspiral | CT: 10 mm; reference to spiral scan deleted |
| Lymph nodes | Not mentioned | Target lesions >15 mm; nontarget lesions 10-15 mm; nonpathologic <10 mm (short-axis measurements) |
| Number of lesions to be measured | 10 lesions (5 per organ) | 5 lesions (2 per organ) |
| Special considerations on lesion measurability | Cystic lesions; bone lesions considered nonmeasurable | Notes included on measurement of bone lesions, cystic lesions |
| RESPONSE CRITERIA | ||
| Target lesions | Complete response: lymph node not mentioned Progressive disease: 20% increase over smallest sum on study or new lesions | Complete response: lymph nodes must be <10 mm in short axis Progressive disease: 20% increase over smallest sum on study (including baseline); at least 5-mm increase or new lesions |
| Nontarget disease | Unequivocal progression considered progressive disease | More detailed description of unequivocal progression; must be representative of overall disease status change, not a single-lesion increase |
| Overall response | Table with integrated target and nontarget lesions | Two tables, one integrating target and nontarget and the other of nontarget only |
| Confirmatory measure | For complete response and partial response: criteria must be met again 4 weeks after initial documentation | Retain this requirement only for nonrandomized trials with primary endpoint of response |
| Progression-free survival | General comments only | More specific comments on use of progression-free survival (or proportion progression free) as phase II endpoint Greater detail on progression-free survival assessment in phase III trials |
| Reporting of response results | Nine categories suggested for reporting of phase II results | Divided into phase II and phase III Nine categories reduced to 5 In phase III, guidance given about reporting response |
| Response in phase III trials | More relaxed guidelines if protocol specified | This section removed; no need to have different criteria for phase II and phase III |
Modifications in RECIST 1.1 include the following: reduced number of target lesions from a total of 10 per patient (and five per organ) to five per patient (and two per organ); classifying lymph nodes as target lesions and measuring their short axis (not the long axis; the long axis is used for other types of target lesions); new definitions of how lesions previously determined to be nonmeasurable (e.g., bone lesions) may now be measured ( Fig. 127-2 ); and modifications of guidelines on imaging modalities to be used.

As in RECIST 1.0, the tumor lesions are categorized as measurable and nonmeasurable on the basis of their size. A measurable lesion is a lesion with longest axial diameter of more than 10 mm by CT scan (with slice thickness not more than 5 mm) and 20 mm by chest radiography. Lymph nodes greater than 15 mm in short axes are considered measurable ( Fig. 127-3 ). Lesions smaller than 10 mm in longest diameter and lymph nodes with short-axis diameter between 11 and 15 mm are considered nonmeasurable. RECIST 1.1 designates several other lesions nonmeasurable. These include small tumors (nodules with a short-axis dimension <10 mm), leptomeningeal disease, lymphangitic spread, inflammatory breast disease, pericardial/pleural effusions, palpable abdominal masses/organomegaly not reproducible on imaging studies, lesions surrounded by postradiation scar tissue, and bone metastases without soft tissue masses measuring more than 10 mm. The presence or absence of nonmeasurable lesions should be noted, as unequivocal progression of these lesions indicates progressive disease. As in RECIST 1.0, it is recommended to have baseline documentation of all target and nontarget lesions.

RECIST 1.1 relies on modalities that are reproducible and accurate. For instance, chest CT is preferred to chest radiography; however, if the lesion is clearly defined on a chest radiograph, it may be considered measurable. Overall, CT is considered the most reliable available method for lesion measurement. Contrast-enhanced MRI may also be used to assess response. Ultrasound, endoscopy, laparoscopy, and tumor markers are considered to be reliably reproducible and not recommended for lesion measurement. PET scan may be helpful in some instances, such as confirming lack of metabolic activity in a site of radiation scar tissue, indicating complete response. New hypermetabolic sites are considered to indicate progressive disease as long as they are confirmed on subsequent CT.
Whereas RECIST 1.1 maintains the four major response categories as in RECIST 1.0, the definition of progressive disease is modified. In addition to a 20% increase in the sum of diameters of target lesions, an absolute increase of at least 5 mm is required in small lesions. Appearance of new lesions is also considered progression. Inclusion of lymph nodes in RECIST 1.1 needs additional clarifications to avoid confusion. If all lymph nodes have a short axis of less than 10 mm, the result is categorized as complete response. This eliminates the major drawback with RECIST 1.0, whereby normal-sized lymph nodes could not be categorized as complete response.
RECIST 1.0 did not have a consensus on how to measure when a lesion splits or when multiple lesions coalesce. In RECIST 1.1, if the lesion splits, the sum of the longest diameters of individual lesions is used and reported as the target lesion sum ( Fig. 127-4 ). If lesions become coalesced and inseparable from one another, the longest diameter of the coalesced lesion is recorded.