Magnetic resonance (MR) imaging plays an integral role in the assessment of articular cartilage. This article discusses the role of MR imaging in the evaluation of articular cartilage, the appearance of cartilage lesions on MR imaging, and the currently available MR imaging techniques for evaluating cartilage morphology. A limitation of currently available sequences is their inability to consistently detect superficial degenerative and posttraumatic cartilage lesions that may progress to more advanced osteoarthritis. In the future, improved image quality may allow for better evaluation of articular cartilage and earlier detection of cartilage lesions.
Osteoarthritis is a highly prevalent chronic disease affecting millions of Americans and is one of the most common causes of disability in the United States. The lifetime risk of developing osteoarthritis of the knee joint has been estimated at 45% with an expected continued increase in prevalence caused by higher rates of obesity and the aging American population. Osteoarthritis of the hip joint is also a common clinical problem, although the prevalence of the disease reported in the literature varies between 1% and 27%. Acute cartilage injury is frequently seen following joint trauma and is a common cause of pain and disability in young and middle-aged patients. With the enormous number of individuals in the United States suffering from the debilitating effects of osteoarthritis and acute cartilage injury, much research has been directed toward developing effective medical and surgical treatment options. Such endeavors require accurate and reproducible methods of cartilage assessment for both clinical and research applications.
Radiographs have been the traditional imaging modality used to evaluate patients with osteoarthritis and acute cartilage injury. However, radiographs can only assess joint space loss and osteophyte formation and are insensitive for detecting early cartilage degeneration. For this reason, magnetic resonance (MR) imaging has become the imaging modality of choice for evaluating articular cartilage in both the clinical and research setting. Because of its high spatial resolution, multiplanar capability, and excellent tissue contrast, MR imaging can detect and characterize morphologic changes associated with cartilage degeneration and acute cartilage injury. Lack of exposure to ionizing radiation is an additional attractive feature of MR imaging, particularly in young patients. This article discusses the role of MR imaging in the evaluation of the articular cartilage of the knee and hip joints, which are the two joints most commonly affected by osteoarthritis and acute cartilage injury. The article also discusses the appearance of cartilage lesions on MR imaging and the advantages and limitations of currently available MR imaging techniques for evaluating cartilage morphology.
Importance of morphologic cartilage assessment
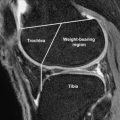
Accurate evaluation of articular cartilage in patients undergoing MR imaging of the knee and hip joints is clinically significant. Identifying focal and diffuse cartilage loss can explain the cause of joint pain in many symptomatic patients. The pain and disability associated with osteoarthritis and acute cartilage injury can also be reduced through early diagnosis and appropriate treatment. Self-management activities such as weight control and physical activity may relieve symptoms and slow the progression of the disease process. New medical therapies may soon become available for the treatment of patients with osteoarthritis and acute cartilage injury. The role of cytokines in the pathophysiology of osteoarthritis has led to the investigation of a multitude of pathways to explain symptoms and structural changes associated with the disease. Numerous agents, including interleukin (IL)-1 receptor antagonist protein, tumor necrosis factor (TNF)-α blockers, calcitonin, and anabolic cytokines, have been evaluated as potential treatment options for patients with osteoarthritis. Early intra-articular administration of hyaluronic acid has also been shown to reduce chondrocyte death and increase proteoglycan content of articular cartilage following acute traumatic injury. Although no pharmaceutical agents are currently approved by the United States Food and Drug Administration for treatment of patients with osteoarthritis or acute cartilage injury, the development of disease-modifying drugs remains an important goal and an area of ongoing research.
Several surgical interventions are currently being used in clinical practice to treat degenerative and posttraumatic cartilage lesions within the knee and hip joints. Surgical procedures such as debridement, microfracture, bone marrow stimulation, autologous osteochondral mosaicplasty, and autologous chondrocyte transplantation have been shown to provide significant pain relief in patients with acute cartilage injury and early osteoarthritis of the knee joint. During the past decade, conditions such as femoroacetabular impingement and acetabular dysplasia have been gaining increased attention as causes of premature osteoarthritis of the hip joint in young patients. Surgical interventions such as labral repair or debridement along with femoral and acetabular osteochondroplasty and acetabular osteotomy are currently available to treat these patients. However, performing these procedures before the development of advanced joint degeneration is essential for their long-term success. Thus, early detection of degenerative and posttraumatic cartilage loss within the knee and hip joints with MR imaging can help identify patients who may benefit from early surgical intervention.
Appearance of degenerative and posttraumatic cartilage lesions on MR imaging
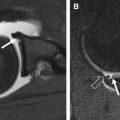
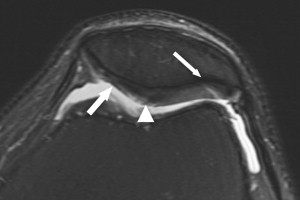
Normal articular cartilage has a smooth contour and multilaminar appearance owing to the presence of several layers with varying amounts of collagen, proteoglycans, and chondrocytes. Degenerative changes within articular cartilage have characteristic features that correlate with the degree of disease severity. Initial changes associated with early cartilage degeneration include foci of fibrillation, fissuring, and surface irregularity ( Fig. 1 ). Diffuse cartilage thinning and cartilage delamination are manifestations of advancing osteoarthritis ( Fig. 2 ). As the disease progresses, partial and full-thickness cartilage defects are commonly seen on opposing articular surfaces. Cartilage defects in the setting of osteoarthritis tend to have obtuse margins and may show increased T2 signal in the underlying subchondral bone marrow ( Fig. 3 ). Additional findings of advanced osteoarthritis include subchondral sclerosis, subchondral cysts, and osteophyte formation, which are all well depicted by MR imaging.
Increased T2 signal resembling bone marrow edema is often seen adjacent to degenerative cartilage lesions and has received attention as an important component in the generation of pain in patients with osteoarthritis. These areas of subchondral bone marrow signal abnormality can be seen adjacent to 5% to 54% of cartilage lesions depending on their size and depth. Although evidence of edema has been found on histologic analysis, additional marrow changes, including alterations in trabecular bone architecture, bone marrow fibrosis, necrosis, and hemorrhage, have been shown, suggesting that edema may play only a minor role in this imaging appearance. For this reason, areas of increased T2 signal adjacent to degenerative cartilage lesions are referred to as the subchondral bone marrow edema pattern. The clinical significance of the subchondral bone marrow edema pattern is controversial. However, recent studies have shown that these areas of signal abnormality correlate with pain and progression of cartilage loss in patients with osteoarthritis. Subchondral sclerosis and bone attrition are also commonly seen in association with advanced degenerative cartilage loss and may reflect osseous remodeling related to altered stress. Loss of mineralized trabecular bone has also been recently recognized as an additional osseous manifestation of advanced osteoarthritis.
Traumatic cartilage lesions tend to have a different appearance than degenerative cartilage lesions on MR imaging and are characterized by sharp and acutely angulated margins ( Fig. 4 ). In addition, traumatic cartilage lesions are often solitary and are found in characteristic locations according to the pattern of injury. Traumatic cartilage lesions are especially common in the knee joint and are the result of tangential, rotational, or shearing forces on the articular surface. The appearance of traumatic cartilage lesions on MR imaging depends on the mechanism of injury.
Tangential and rotational forces on articular cartilage secondary to injuries such as anterior cruciate ligament tear result in osteochondral impaction injuries. These impaction injuries are secondary to a pivot shift mechanism and manifest as areas of high T2 signal within the subchondral bone marrow of the anterior lateral femoral condyle and posterior tibial plateau with occasional depression of the articular surface and trabecular fracture lines ( Fig. 5 ). These areas of bone marrow signal abnormality correlate with areas of significantly increased ( P <.05) water and unsaturated lipid content and significantly decreased ( P <.05) saturated lipid content on MR imaging spectroscopy. Detection of high T2 signal within the subchondral bone marrow in the setting of acute joint injury should lead to careful scrutiny of the overlying articular cartilage. At MR imaging and arthroscopy, the articular cartilage overlying areas of subchondral bone marrow edema typically appears normal. However, biochemical changes of acute cartilage injury may be detected on histologic analysis or when using advanced imaging techniques such as delayed gadolinium-enhanced MR imaging and T1-rho imaging. Areas of subchondral bone marrow edema may persist for several months following acute traumatic injury, although their long-term clinical significance remains unknown.
Shearing forces on articular cartilage secondary to injuries such as transient patellar dislocation typically result in deep partial-thickness or full-thickness cartilage defects with acutely angulated margins that may also involve the underlying subchondral bone. Cartilage flap tears and cartilage delamination from the underlying subchondral bone plate may also occur. In the setting of transient patellar dislocation, the injury pattern is typically seen on the medial patellar facet and anterior lateral femoral condyle ( Fig. 6 ). Cartilage shearing injuries are often unstable and may result in intra-articular cartilaginous or osteochondral loose bodies, which may lead to clinical symptoms such as locking that may mimic displaced meniscal tears.
Appearance of degenerative and posttraumatic cartilage lesions on MR imaging
Normal articular cartilage has a smooth contour and multilaminar appearance owing to the presence of several layers with varying amounts of collagen, proteoglycans, and chondrocytes. Degenerative changes within articular cartilage have characteristic features that correlate with the degree of disease severity. Initial changes associated with early cartilage degeneration include foci of fibrillation, fissuring, and surface irregularity ( Fig. 1 ). Diffuse cartilage thinning and cartilage delamination are manifestations of advancing osteoarthritis ( Fig. 2 ). As the disease progresses, partial and full-thickness cartilage defects are commonly seen on opposing articular surfaces. Cartilage defects in the setting of osteoarthritis tend to have obtuse margins and may show increased T2 signal in the underlying subchondral bone marrow ( Fig. 3 ). Additional findings of advanced osteoarthritis include subchondral sclerosis, subchondral cysts, and osteophyte formation, which are all well depicted by MR imaging.
Increased T2 signal resembling bone marrow edema is often seen adjacent to degenerative cartilage lesions and has received attention as an important component in the generation of pain in patients with osteoarthritis. These areas of subchondral bone marrow signal abnormality can be seen adjacent to 5% to 54% of cartilage lesions depending on their size and depth. Although evidence of edema has been found on histologic analysis, additional marrow changes, including alterations in trabecular bone architecture, bone marrow fibrosis, necrosis, and hemorrhage, have been shown, suggesting that edema may play only a minor role in this imaging appearance. For this reason, areas of increased T2 signal adjacent to degenerative cartilage lesions are referred to as the subchondral bone marrow edema pattern. The clinical significance of the subchondral bone marrow edema pattern is controversial. However, recent studies have shown that these areas of signal abnormality correlate with pain and progression of cartilage loss in patients with osteoarthritis. Subchondral sclerosis and bone attrition are also commonly seen in association with advanced degenerative cartilage loss and may reflect osseous remodeling related to altered stress. Loss of mineralized trabecular bone has also been recently recognized as an additional osseous manifestation of advanced osteoarthritis.
Traumatic cartilage lesions tend to have a different appearance than degenerative cartilage lesions on MR imaging and are characterized by sharp and acutely angulated margins ( Fig. 4 ). In addition, traumatic cartilage lesions are often solitary and are found in characteristic locations according to the pattern of injury. Traumatic cartilage lesions are especially common in the knee joint and are the result of tangential, rotational, or shearing forces on the articular surface. The appearance of traumatic cartilage lesions on MR imaging depends on the mechanism of injury.
Tangential and rotational forces on articular cartilage secondary to injuries such as anterior cruciate ligament tear result in osteochondral impaction injuries. These impaction injuries are secondary to a pivot shift mechanism and manifest as areas of high T2 signal within the subchondral bone marrow of the anterior lateral femoral condyle and posterior tibial plateau with occasional depression of the articular surface and trabecular fracture lines ( Fig. 5 ). These areas of bone marrow signal abnormality correlate with areas of significantly increased ( P <.05) water and unsaturated lipid content and significantly decreased ( P <.05) saturated lipid content on MR imaging spectroscopy. Detection of high T2 signal within the subchondral bone marrow in the setting of acute joint injury should lead to careful scrutiny of the overlying articular cartilage. At MR imaging and arthroscopy, the articular cartilage overlying areas of subchondral bone marrow edema typically appears normal. However, biochemical changes of acute cartilage injury may be detected on histologic analysis or when using advanced imaging techniques such as delayed gadolinium-enhanced MR imaging and T1-rho imaging. Areas of subchondral bone marrow edema may persist for several months following acute traumatic injury, although their long-term clinical significance remains unknown.
Shearing forces on articular cartilage secondary to injuries such as transient patellar dislocation typically result in deep partial-thickness or full-thickness cartilage defects with acutely angulated margins that may also involve the underlying subchondral bone. Cartilage flap tears and cartilage delamination from the underlying subchondral bone plate may also occur. In the setting of transient patellar dislocation, the injury pattern is typically seen on the medial patellar facet and anterior lateral femoral condyle ( Fig. 6 ). Cartilage shearing injuries are often unstable and may result in intra-articular cartilaginous or osteochondral loose bodies, which may lead to clinical symptoms such as locking that may mimic displaced meniscal tears.
Morphologic cartilage imaging sequences
Techniques and approaches to MR imaging assessment of articular cartilage have changed rapidly in the past 2 decades. Various MR imaging pulse sequences have been successfully used to evaluate articular cartilage. These sequences include two-dimensional and three-dimensional fast spin echo sequences, three-dimensional gradient-echo sequences, dual echo in the steady-state (DESS) sequence, driven equilibrium Fourier transform sequence, and various steady-state free-precession sequences. The fact that so many MRI techniques exist for evaluating articular cartilage illustrates the challenges of accurate diagnosis and the inherent limitations of each imaging strategy.
Two-dimensional and Three-dimensional Fast Spin Echo Sequences
Two-dimensional fast spin echo (2D-FSE) sequences with intermediate-weighted and T2-weighted contrast are the most common sequences used in clinical practice to evaluate articular cartilage. 2D-FSE sequences have high in-plane spatial resolution and can be used to evaluate the menisci, ligaments, and osseous structures in addition to articular cartilage. However, 2D-FSE sequences have thick slices and gaps between slices, which may limit visualization of small cartilage lesions secondary to partial volume averaging. Furthermore, T2-weighted 2D-FSE sequences create contrast between cartilage and synovial fluid at the expense of cartilage signal. Although cartilage visibility on these sequences is partially limited by the magnetization transfer effect secondary to multiple off-resonance pulses, magnetization transfer contrast may also be clinically useful. Intermediate-weighted 2D-FSE sequences have suboptimal contrast between cartilage and synovial fluid and suffer from image blurring caused by the acquisition of high spatial frequencies late in the echo train. However, contrast between cartilage and synovial fluid can be improved with the addition of fat suppression, whereas image blurring can be reduced with the use of short echo train lengths.
Recent research has brought attention to the use of three-dimensional fast spin echo (3D-FSE) sequences for evaluating articular cartilage such as fast spin echo Cube (FSE-Cube; GE Healthcare) and sampling perfection with application-oriented contrasts using different flip angle evolutions (SPACE, Siemens Medical Systems). 3D-FSE sequences use variable flip angle modulation to constrain T2 decay for an extended echo train, which allows intermediate-weighted images with bright synovial fluid to be acquired with minimal blurring. A major advantage of these sequences is their ability to acquire volumetric datasets with isotropic resolution, which allow articular cartilage to be evaluated in any orientation following a single acquisition. 3D-FSE sequences can also be used to evaluate the menisci, ligament, and osseous structures in addition to articular cartilage. 3D-FSE sequences have higher cartilage signal/noise ratio (SNR) but lower contrast between cartilage and synovial fluid compared with 2D-FSE sequences. Magnetization transfer contrast is diminished on 3D-FSE sequences, which helps preserve cartilage signal but may potentially decrease the conspicuity of early cartilage degeneration. In-plane spatial resolution is also lower on 3D-FSE sequences compared with 2D-FSE sequences unless long acquisition times are used, which may also limit the detection of superficial cartilage lesions.
Three-dimensional Gradient-Echo Sequences
Gradient-echo sequences were the first three-dimensional sequences used for cartilage imaging. Gradient-echo sequences can be divided into dark fluid sequences and bright fluid sequences based on the signal intensity of synovial fluid. Dark fluid sequences include T1-weighted spoiled gradient-recalled echo (SPGR; GE Healthcare), fast low-angle shot (FLASH; Siemens Medical Systems), and T1 fast field echo (T1-FFE; Philips Healthcare). Bright fluid gradient-echo sequences include T2*-weighted gradient-recalled echo acquired in the steady state (GRASS; GE Healthcare), gradient-recalled echo (GRE; Siemens Medical Systems), and fast field echo (FFE; Philips Healthcare). Fat suppression is typically added to gradient-echo sequences to reduce chemical shift artifact and to optimize the overall dynamic contrast range of the image. Frequency-selective fat saturation is the most commonly used method to suppress fat signal. However, gradient-echo images with higher SNR and greater contrast between cartilage and adjacent joint structures can be obtained using recently developed fat-suppression techniques such as water excitation and iterative decomposition of water and fat with echo asymmetry and least squares estimates (IDEAL).
Both dark fluid and bright fluid three-dimensional gradient-echo sequences have been successfully used to evaluate articular cartilage in clinical practice and to perform cartilage volume measurements in osteoarthritis research studies. However, the main disadvantage of using dark fluid sequences for clinical cartilage imaging is the low signal intensity of synovial fluid, which may decrease the conspicuity of superficial cartilage lesions ( Fig. 7 ). Dark fluid sequences have lower contrast between articular cartilage and synovial fluid than bright fluid sequences. In addition, the surface properties of degenerative cartilage may influence the ability of three-dimensional sequences to detect superficial cartilage lesions. Superficial degeneration shortens the T2 relaxation time of cartilage. For dark fluid sequences, the T2 shortening of degenerative cartilage has no effect on its signal intensity and contrast relative to synovial fluid. However, for bright fluid sequences, the effect of T2 shortening is to decrease the signal intensity of degenerative cartilage and thus increase its contrast relative to synovial fluid, which may result in greater conspicuity of superficial cartilage lesions.
Three-dimensional DESS Sequence
DESS is another three-dimensional sequence that has been used to evaluate articular cartilage. The DESS sequence acquires 2 gradient echoes separated by a refocusing pulse that are combined into a single image. Adding the 2 echoes enhances the T2*-weighting of the image and increases the signal intensity of both cartilage and synovial fluid. Although the DESS sequence has primarily been performed with a flip angle of less than 60°, some investigators have proposed the use of a larger flip angle to further accentuate fluid signal and improve contrast between cartilage and synovial fluid. Cartilage SNR and contrast between cartilage and synovial fluid can also be improved by an individualized weighting procedure instead of the conventional averaging of the 2 gradient-echo images. In a recent study comparing multiple three-dimensional isotropic resolution sequences for evaluating the articular cartilage of the knee joint at 3.0T, a water-excitation DESS sequence with 0.5-mm isotropic resolution and an 8-minute scan time was found to have the highest contrast between cartilage and synovial fluid and the greatest overall performance on qualitative assessment. A water-excitation DESS sequence with near isotropic resolution is also being used in the Osteoarthritis Initiative to evaluate the articular cartilage of the knee joint.
Three-dimensional Driven Equilibrium Fourier Transform
Driven equilibrium Fourier transform (DEFT) and variants such as fast recovery fast spin echo and driven equilibrium (DRIVE) are additional three-dimensional cartilage imaging sequences. The DEFT sequence differs from other techniques in that a 90° pulse is used to return transverse magnetization to the z-axis which increases signal from tissues such as synovial fluid that have long T1 relaxation times. The result is an image that is dependent on the ratio of T1/T2 signal rather than traditional T1-weighting or T2-weighting. The DEFT sequence can create high-resolution three-dimensional images of the knee joint with bright synovial fluid when combined with a three-dimensional echo-planar readout. Cartilage signal is preserved by the use of a short echo time. The DEFT sequence has similar cartilage SNR to 2D-FSE and fat-suppressed SPGR sequences with as much as 4 times greater contrast between cartilage and synovial fluid. The bright synovial fluid on DEFT images creates an arthrogramlike effect that may potentially increase the conspicuity of superficial cartilage lesions.
Three-dimensional Balanced Steady-state Sequences
Balanced steady-state free-precession (SSFP) sequences are additional three-dimensional sequences that have been used for evaluating articular cartilage. Balanced SSFP sequences include commercially available sequences such as fast imaging using steady-state acquisition (FIESTA; GE Healthcare), true fast imaging with steady-state precession (true-FISP; Siemens Medical Systems), and balanced fast field echo imaging (balanced-FFE; Philips Healthcare), and variants such as fluctuating equilibrium magnetic resonance (FEMR), vastly undersampled isotropic projection steady-state free precession (VIPR-SSFP), and alternating repetition time vastly undersampled isotropic projection steady-state free precession (VIPR-ATR). Balanced SSFP sequences can be combined with various methods of fat suppression such as water excitation, linear combination, intermittent frequency-selective fat saturation, phase detection, and IDEAL. These sequences have higher cartilage SNR and higher contrast between cartilage and synovial fluid than 2D-FSE and three-dimensional fat-saturated SPGR sequences. Balanced SSFP sequences also have highly versatile tissue contrast that can be used to evaluate the menisci, ligament, and osseous structures in addition to articular cartilage.
The use of balanced SSFP sequences for evaluating articular cartilage has increased in recent years because of improvements in MR imaging technology that have reduced artifacts associated with eddy current–induced signal distortions and off-resonance effects. However, banding artifacts caused by off resonance are still problematic when using long repetition times and high field strength scanners. To reduce banding artifacts, repetition times for balanced SSFP sequences are typically kept to less than 10 milliseconds, which ultimately limits spatial resolution. Balanced SSFP sequences with multiple acquisitions can be used to achieve higher resolution at the expense of additional scan time.
The VIPR-SSFP sequence is a balanced SSFP variant that uses a highly SNR-efficient, dual-echo, radial k-space trajectory to improve spatial resolution while maintaining clinically feasible scan times. Unlike Cartesian methods, VIPR-SSFP sequence does not require phase encoding, slice encoding, or dephasing gradients, which allows for almost continuous acquisition of data. The sequence uses a linear combination method to separate signal from fat and water. At 1.5T, the optimal repetition time for linear combination fat-water separation is 2.4 milliseconds, which can be achieved while maintaining adequate time to allow for spatial encoding. However, linear combination balanced SSFP sequences such as VIPR-SSFP are difficult to implement at 3.0T because of limitations on the time available for spatial encoding with the increase in magnetic field strength. At 3.0T, linear combination fat-water separation is performed by using a repetition time of 3.6 milliseconds and skipping a fat passband. The skipped passband increases the sensitivity to magnetic field inhomogeneity. However, the VIPR-SSFP sequence exploits the phase progression between the 2 half-echoes acquired within each repetition time to improve fat-water separation and reduce the sensitivity to off-resonance artifacts. The VIPR-SSFP sequence can acquire multiplanar, fat-suppressed images of the knee joint with 0.5-mm isotropic resolution at 1.5T and 0.4-mm isotropic resolution at 3.0T following a single 5-minute acquisition.
The VIPR-ATR sequence is another balanced SSFP variant that uses the highly SNR-efficient, dual-echo VIPR radial k-space trajectory. The VIPR-ATR sequence uses 2 different alternating length repetition times and radiofrequency phase cycling to create a null for off-resonance fat signal during the balanced SSFP acquisition. Compared with VIPR-SSFP, the VIPR-ATR sequence offers enhanced image quality with comparable fat suppression because its single k space acquisition allows for a reduction in undersampling artifact. Furthermore, the ability to image on-resonance avoids the image blurring associated with the VIPR-SSFP sequence when higher resolution requires longer data acquisition intervals. VIPR-ATR can acquire multiplanar, fat-suppressed images of the knee joint with 0.3-mm isotropic resolution at 3.0T following a single 8-minute acquisition ( Fig. 8 ). In contrast, the water-excitation DESS sequence used in the Osteoarthritis Initiative produces images of the knee joint with 0.4×0.5×0.7-mm voxel size at 3.0T with an acquisition time of more than 10 minutes.