Inflammatory bowel disease (IBD) is an important cause of abdominal pain in the pediatric population. Magnetic resonance enterography (MRE) plays a crucial role in assessment of disease severity, location, extent of disease, and assessment for associated complications. As MR imaging technology has advanced, new techniques have been brought into clinical practice. Recent research has expanded our understanding of how the inflammatory processes in IBD manifest on MRE and improved radiology’s ability to accurately assess the disease and its associated complications. This article discusses up-to-date MR imaging techniques and imaging manifestations of IBD in pediatric patients.
Key points
- •
Inflammatory bowel disease (IBD) is an important cause of abdominal pain in the pediatric population.
- •
MR imaging/MRE (magnetic resonance enterography) is a valuable imaging modality in assessment of IBD disease location and extent.
- •
MR imaging/MRE technology continues to evolve and advance with new techniques and imaging pulse sequences that improve disease detection and evaluation.
- •
MR imaging/MRE is a valuable tool to help assess intra-abdominal extraintestinal manifestations of IBD.
Introduction
Pediatric inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is an important cause of gastrointestinal pathology in children and adolescents. Although the incidence of IBD in children is less than adults, there are indications that the incidence is increasing in the pediatric population. As many as 30% of patients with CD and 20% of patients with UC are younger than 20 years. As a result of rising incidence, radiologists interpreting MR imaging can expect to encounter pediatric IBD with increasing frequency and must be familiar with disease manifestations.
The radiologist also must have an appropriate imaging strategy in place for evaluating pediatric IBD. The strength of magnetic resonance enterography (MRE) lies in the excellent soft tissue contrast, permitting detailed evaluation of the bowel wall, mesentery, and regional soft tissues, while not exposing children to the potentially harmful effects of ionizing radiation. MRE is an appropriate tool for evaluating children with newly diagnosed IBD, those with known disease and acute symptoms, and those with known disease who require routine monitoring.
Ensuring an up-to-date understanding and working knowledge of the currently available tools and techniques necessary for MRE is imperative to implementing best practices in IBD imaging, as ever-advancing MR imaging technology continues to permit improved pediatric abdominopelvic imaging capabilities. This article provides an up-to-date review of imaging technique and the current understanding of imaging manifestations in pediatric IBD.
Imaging technique
Patient Preparation
Appropriate patient preparation is an important initial step in optimization of the pediatric MR image for evaluation of IBD. The following sections discuss the necessary components of patient preparation.
Sedation
Cooperation for MR imaging can be challenging in patients younger than 6 to 7 years old, patients with claustrophobia, and patients with developmental delay. Nonenterography MR imaging can be performed in these patient populations using sedation or general anesthesia. However, the necessity of ingesting oral contrast to ensure adequate distention of the small bowel for MRE conflicts with the requirement to avoid ingestion of any food or drink within 6 hours when sedation and/or general anesthesia is required. Using a combination of child life services, video goggles, and an abbreviated protocol may render performance of a nonsedated MRE in younger children (4–7 years of age) feasible. Those unable to tolerate the examination and comply with MR imaging technologist instructions have to be evaluated with an alternative imaging method, such as computed tomography (CT) or small-bowel-follow-through.
Alternatively, in concert with the anesthesiologist, the radiologist may elect to place a nasogastric tube and administer enteric contrast over a defined period with the patient under anesthesia, as described by Mollard and colleagues. The radiologist should remain aware of the potential risks of anesthesia in pediatric patients, including immediate effects such as cardiovascular and pulmonary compromise and potential long-term neurodevelopmental and cognitive impairment. Incorporation of these risk considerations into a best imaging strategy for the individual pediatric patient is crucial.
Oral contrast
Ingestion of oral contrast is an integral step in patient preparation for MR imaging evaluation of IBD. Consumption of a biphasic (hypointense on T1-weighted images and hyperintense on T2-weighted images) contrast agent, such as the barium-containing contrast material VoLumen (Bracco Diagnostics, Princeton, NJ), helps ensure adequate bowel distention permitting a more thorough assessment of the bowel. In particular, adequate distention helps alleviate false-positive impressions of bowel wall thickening and luminal narrowing. As this material is also hypointense on T1-weighted images, abnormalities of mucosal enhancement become more conspicuous on T1-weighted postcontrast images.
Generally, pediatric patients are asked to consume 900 mL of oral contrast material over the 45 minutes preceding examination initiation. Patients requiring sedation or anesthesia for the examination may require the placement of a nasogastric tube and direct administration of the contrast agent into the stomach over several minutes before the examination initiation. Those patients who refuse to drink oral contrast may consume water, as tolerated, as they frequently have severe enough disease to permit disease detection without optimal distention.
Glucagon
Spasmolytic agents, such as glucagon, may be used in pediatric MRE to help reduce motion artifact related to bowel peristalsis. Glucagon can be administered by intramuscular or intravenous injection as a single 0.5-mg dose (either at the beginning of the examination or just before contrast administration) or as a split dose (0.25 mg before precontrast images are acquired and 0.25 mg before intravenous [IV] contrast is given). Common side effects from administration of glucagon include nausea and vomiting, particularly when administered IV. These side effects may be partially mitigated by proper hydration and slow administration of the glucagon injection when given IV. In addition, the radiologist should be aware of contraindications to administration of glucagon that include prior allergy to glucagon, diabetes mellitus, pheochromocytoma, adrenal insufficiency, and insulinoma.
Intravenous Contrast
IV injection of gadolinium-based contrast agents (GBCAs) is recommended to improve detection and assessment of diseased bowel segments and associated disease-related complications, including intra-abdominal abscesses and fistulizing disease. IV contrast agents also assist in assessment of extraintestinal disease-related complications. GBCAs are generally injected at a dose of 0.2 mL/kg (up to 20 mL when using a standard agent) at a rate of 1 to 2 mL/s via a peripheral IV catheter. GBCAs should be avoided in those patients with contraindications to administration that include history of prior moderate or severe allergiclike reaction to gadolinium-based agents and acute kidney injury/chronic renal disease.
Technical Preparation
Coil selection
Field-of-view (FOV) coverage should extend from the lung bases through the level of the pubic symphysis, ensuring coverage of the perineal region. Flexible phased array torso/body coils, in addition to the spine coil built into the MR imaging table, can be used for abdomen and pelvis coverage.
MR imaging sequence and protocol selection
MRE may be performed on either 1.5-T or 3.0-T MR imaging systems, with each field strength offering advantages and disadvantages. Generally, artifacts due to bowel gas and intra-abdominal surgical material are more significant at 3.0 T compared with 1.5 T. However, 3.0 T offers improved signal-to-noise and contrast-to-noise ratios, as well as superior spatial resolution. Generally, a combination of rapid axial and coronal fluid-sensitive sequences are acquired before contrast administration. Commonly used MR imaging sequences are single-shot fast spin-echo (SSFSE) and balanced steady-state free-precession (BSSFP), both of which are motion resilient and nicely depict bowel and mesentery anatomy. Fat suppression excellently shows the presence of inflammation, edema, and fluid. Diffusion-weighted-imaging (DWI) can be used to help localize inflamed bowel segments, particularly in those patients who cannot receive IV contrast. Typically, single-shot echo-planar-based pulse sequences are used and increasing the number of signal averages with the patient free-breathing or using breath-hold or respiratory triggered imaging help decrease conspicuity of respiratory motion artifact. Cine MRE can provide functional information regarding bowel peristalsis and is performed using the BSSFP sequence. Precontrast T1-weighted fat-suppressed sequences, most often 2-dimensional or 3-dimensional (3D) gradient recalled echo (GRE), also are a necessary component of the protocol.
Dynamic contrast-enhanced imaging is a very importance part of the MRE protocol. Dynamic imaging may help improve detection and characterization of diseased bowel and generally is performed with image acquisition at 45 to 55 seconds after contrast administration (enteric phase), 60 to 75 seconds (portal venous phase), and approximately 180 seconds after contrast administration. Newer non-Cartesian T1-weighted GRE sequences (eg, Star-VIBE; Siemens Healthcare, Erlangen, Germany) to help reduce motion artifacts, such as respiratory motion and bowel peristalsis motion, can be used for precontrast and postcontrast images in the MRE protocol to supplement the dynamic postcontrast images.
Imaging evaluation
Intestinal Manifestations of Inflammatory Bowel Disease
Differentiating between CD and UC on the basis of imaging alone may not be possible, and the radiologist should remain aware that significant overlap of imaging findings can be present. However, characteristic distributions and patterns of disease may suggest one diagnosis over the other, and the following discussion reviews some common imaging findings and complications of each disease entity.
Crohn disease
CD may affect any segment of the gastrointestinal tract, and the patient’s presentation is often determined by the location and extent of disease involvement. The jejunum, ileum, and colon are well assessed by MRE. Although the esophagus, stomach, and duodenum can be evaluated with MR imaging, they are better assessed with endoscopy. The most commonly affected sites are the terminal ileum, ileocecal region, and colon. CD is characterized by aphthous ulceration that may lead to a cobblestone pattern when deep ulcers coalesce and result in islands of normal mucosa interspersed with ulcerated mucosa. The inflammation may extend through the entire thickness of the bowel wall. The foci of inflammation may be interspersed with normal segments of bowel, resulting in the so-called “skip lesions.” The inflammation may extend to involve the adjacent mesentery and regional lymph nodes.
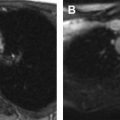
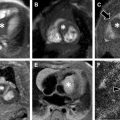
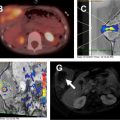
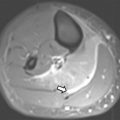
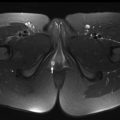
MR imaging findings in CD reflect the observed pathologic processes and associated complications. The radiologist should carefully evaluate the enteric system, and describe the location, length, and imaging characteristics of the inflamed bowel segment(s). The 3 most common findings in active CD are bowel wall thickening, edema, and hyperenhancement. Bowel wall thickening can have a variety of appearances, but typically manifests as a wall thickness greater than 3 mm and may involve the entire circumference of the bowel wall universally or eccentrically. Asymmetric inflammation along the mesenteric border is probably specific for CD. Bowel wall edema manifests as increased intramural signal intensity on T2-weighted sequences ( Fig. 1 ). Dynamic postcontrast imaging may demonstrate segmental hyperenhancement of bowel wall on early (arterial) phase imaging indicative of active inflammation or minimal early enhancement and more homogeneous delayed enhancement indicative of less active and more chronic CD ( Fig. 2 ). DWI has been shown capable of identifying abnormal bowel segments that show restricted DWI and reduced apparent diffusion coefficient within the inflamed segments.


Small bowel luminal narrowing may be observed, either on the basis of acute inflammation or fibrosis. Strictures are areas of persistent luminal narrowing associated with proximal dilation ( Fig. 3 ). The radiologist should evaluate for upstream dilation of bowel and assess the peristaltic movements of the involved bowel segments if Cine images are available. Detection of strictured and/or fibrotic segments of bowel are important to document, as they may require surgical resection. Although a number of MRE findings have been correlated with the presence of fibrosis, most are relatively unreliable and nonspecific because there is often coexistent inflammation and fibrosis in the same segment of bowel, which can have overlapping imaging features. Upstream dilation of bowel 3 cm or more has shown the best correlation with histologically documented fibrosis at the time of resection.

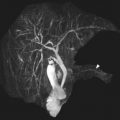
Multiple imaging findings beyond the bowel that support a diagnosis of CD may be encountered. Bowel segments affected by CD may have engorged mesenteric vascularity (vasa recta), referred to as the “comb sign,” that may be evident on fluid-sensitive sequences and postcontrast T1-weighted images ( Fig. 4 ). There may be edema and enlarged lymph nodes within the affected bowel mesentery that become evident on the fluid-sensitive sequences. Focally increased mesenteric fibrofatty tissue (fibrofatty proliferation or “creeping fat”) may be seen adjacent to segments of bowel affected by CD. Such focally increased mesenteric fibrofatty tissue typically manifests on MR imaging as separated bowel loops with asymmetrically increased fat that has similar imaging characteristics to subcutaneous fat and suppressed signal on fat-suppressed images.