MR imaging is frequently used to assess pediatric genital disorders. The ability to obtain 3-dimensional sequences allowing for multiplanar reformations and sequences designed to minimize motion artifact have aided in the imaging evaluation in the pediatric population. For certain genital disorders, such as Müllerian duct anomalies, MR imaging has become the standard imaging technique. This article discusses up-to-date MR imaging techniques and the interpretation of findings including normal anatomy, as well as congenital and acquired genital disorders seen in the pediatric population.
Key points
- •
Pediatric genital disorders encompass a variety of conditions, which may be congenital or acquired.
- •
Congenital conditions include disorders of sex development and Müllerian duct anomalies and some acquired conditions in the pediatric population include cysts and malignancy.
- •
MR imaging is considered the standard modality in the evaluation of Müllerian duct anomalies.
- •
MR imaging provides superior soft tissue contrast, multiplanar capabilities, and is not limited by patient attributes such as body habitus or bowel gas and stool.
- •
MR imaging is often performed to obtain additional information in the evaluation of genital tumors such as extent of disease and tissue characteristics.
Introduction
The ability to image genital disorders in children has undergone significant advancement over the years. Although ultrasound remains the favored modality for the initial evaluation of pediatric patients with a suspected genital abnormality, ultrasound imaging is limited to the transabdominal and/or transperineal approach for most pediatric patients. Ultrasound examination may also be limited by bowel gas and stool, as well as the body habitus of the patient. Fluoroscopy (genitography) is useful in assessing perineal orifices including the presence or absence of the vagina and its relationship with the urethra. However, this technique is invasive and requires ionizing radiation. Computed tomography scanning is little used in the evaluation of pediatric genital disorders owing to the ionizing radiation and inferior soft tissue contrast.
MR imaging has become an invaluable tool for the further assessment of the uterus and genitalia and is useful in the evaluation of associated intraabdominal abnormalities. MR imaging allows for multiplanar imaging with excellent soft tissue resolution. Significant progress has been made with MR imaging with respect to abdominal and pelvic imaging in children. Faster sequences and motion correction techniques help to address the challenges of patient motion. Stronger field strengths and a variety of sequences allow for the improved differentiation of tissue type.
In this article, current MR imaging techniques are described for the evaluation of genital disorders which occur in the pediatric population. Given space constraints, congenital genital abnormalities are limited to Müllerian duct anomalies (MDAs) and disorders of sex differentiation (DSDs) and acquired abnormalities focus on malignancy and cysts.
MR imaging techniques
Patient Preparation
There are many challenges unique to children with respect to MR imaging. Owing to the relatively long acquisition times, patient motion is a challenge. Even the most compliant children may have difficulty staying still for the duration of the study. Child life specialists, MR imaging-compliant video goggles, or child-friendly scanners can help to decrease anxiety and increase tolerance.
In general, most children under 4 years of age require some form of anesthesia. Most young pediatric patients are able to undergo moderate sedation without a need for general anesthesia. If evaluation is required in a newborn, often feeding then swaddling the baby immediately before the study will suffice.
MR Imaging Protocols
Each institution has slightly different variations in their imaging protocols ( Table 1 ). Protocols also need to be tailored to answer the clinical question. Imaging of the pelvis should always include a fluid-sensitive sequence such as T2-weighted sequences with or without fat suppression. Many institutions obtain this as a 3-dimensional (3D) sequence that allows for multiplanar reformations. Additional sequences including the abdomen can be obtained to evaluate for associated abnormalities such as renal anomalies with MDAs.
| Sequence | Use | Notes |
|---|---|---|
| T1W images | For the detection of fat, blood products, and mucinous/proteinaceous content, precontrast and postcontrast imaging | Conventional sequences are easily degraded by motion |
| T2W images | Best visualizes cysts, other fluid-containing structures, uterine zonal anatomy, vaginal anatomy, and gonads | Obtain coronal-oblique orientation through the uterus to assess external contour and uterine cavity Obtain sagittal and axial oblique orientations through vagina |
| 3D T2W images | Useful in imaging small anatomic structures, allows for multiplanar reformations | Can provide higher spatial resolution and signal-to-noise ratio than 2D counterparts |
| Motion correction sequences | Helps reduce motion artifacts seen in conventional T1W and T2W Cartesian sequences | Longer acquisition times than their conventional counterparts |
Intravenous contrast material is generally not necessary for the routine assessment of congenital genital disorders. However, it is helpful in the evaluation of genital tumors and infection. Macrocyclic gadolinium-based contrast agents are considered more stable with less tissue deposition of gadolinium than their linear counterparts and many pediatric institutions currently have switched to macrocyclic agents.
T1-weighted imaging
T1-weighted imaging is essential in the evaluation of fat and blood products. This sequence is especially useful in cyst characterization or evaluating retained blood within the uterus, cervix, and vagina. Proteinaceous or subacute blood products are T1 hyperintense. However, conventional T1-weighted sequences such as turbo or fast spin-echo T1-weighted sequences are easily degraded by motion, a common problem in the pediatric population.
T2-weighted imaging
Cysts, other fluid-containing structures, and gonads are best seen on T2-weighted sequences, although a wider field of view to include the abdomen may be needed if gonads are not identified in the typical positions. The uterus is also best evaluated on a T2-weighted sequence, especially in an oblique coronal plane parallel to the uterine cavity; this sequence allows for the assessment of the fundal contour and shows the junctional zonal anatomy best.
High-resolution T2-weighted imaging
Three-dimensional sequence (trade names: SPACE [sampling perfection with application optimized contrasts using different flip angle evolution], Siemens Healthcare, Erlangen, Germany; CUBE, GE Healthcare, Waukesha, WI; VISTA [volume isotropic turbo spin echo acquisition], Philips Healthcare, Best, the Netherlands; isoFSE [isotropic fast spin echo], Hitachi Medical Systems America, Twinsburg, OH; and 3D MVOX [multivoxel], Toshiba America Medical Systems, Tustin, CA) acquisition allows for reformations through many planes, which can decrease the overall imaging time. In addition, 3D T2-weighted sequences can provide higher spatial resolution and signal-to-noise ratio than their 2-dimensional counterparts, especially at 3T. The 3D T2-weighted volumetric acquisition takes several minutes, however, because 1-mm contiguous slices are acquired; respiratory triggering or navigator gating may be needed when obtaining in children. Because contiguous, thin slices are acquired, this sequence is useful in the imaging of small anatomic structures.
Motion correction techniques
MR image degradation from patient motion is a challenge in the pediatric population. A non-Cartesian sequence such as PROPELLER (periodically rotating overlapping parallel lines with enhanced reconstruction; GE Healthcare), BLADE (Siemens Healthcare), or MultiVane (Philips Healthcare) can help to reduce motion artifacts. Non-Cartesian methods are available for both T1- and T2-weighted sequences. Unfortunately, the non-Cartesian sequences do not allow for dynamic postcontrast imaging given the longer acquisition time.
Image interpretation
Normal Anatomy
Female
Uterus and cervix
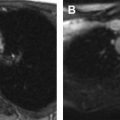
The prepubertal uterus is approximately 2 to 4 cm in length and is tubular in configuration with the anterior–posterior thickness of the fundus equal to that of the cervix. The prepubertal endometrium is very thin and difficult to see. There is an exception during the neonatal period, however, owing to in utero maternal estrogen exposure where the endometrium can demonstrate characteristics closer to the postpubertal uterus. The neonatal cervix is also twice the size of the uterus with the anterior–posterior thickness of the fundus equal to or less than that of the cervix ( Fig. 1 ).

During puberty, the uterus enlarges (reaching a normal length of 5–8 cm) and the fundus becomes more bulbous in contour. After puberty, the trilaminar zonal anatomy becomes readily apparent on T2-weighted images ( Fig. 2 ). The outer layer of the myometrium is intermediate to slightly hyperintense on T2-weighted images; flow voids may be seen from prominent arcuate vasculature. The junctional zone (inner layer of the myometrium) is generally T2 hypointense owing to a low water content and the presence of smooth muscle fibers. The endometrium undergoes cyclical changes with menses but remains high in signal on T2-weighted images owing to the presence of mucinous endometrial glands. Because the uterus is uniform in signal on T1-weighted images, this sequence is of limited use for the evaluation of uterine zonal anatomy.

The cervix is composed of 3 layers—the central zone with a surrounding cervical stroma composed of an inner and outer zone. The central zone is T2-hyperintense owing to the mucinous endocervical glands. The inner zone demonstrates low T2 signal and the outer zone has an intermediate T2 signal.
Ovaries
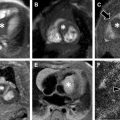
Follicles are present within the ovaries from birth. The follicles’ hyperintense signal on T2-weighted images allows for easy identification on MR imaging. The normal central ovarian medulla is hyperintense on T2-weighted imaging compared with that of the relatively hypointense cortex. Ovarian volume during the neonatal period averages just over 1 mL; however, owing to maternal estrogen influence, follicular cysts can become quite large ( Fig. 3 ). After the first year of life, the ovaries maintain small immature follicles. Between approximately 7 and 12 years of age, the ovaries double in size ( Fig. 4 ). Postpubertal ovaries can contain a corpus luteum usually seen as a cystic structure less than 3 cm in diameter ( Fig. 5 ). The corpus luteum has a thick wall that demonstrates an intermediate to low signal on T2-weighted images. If the corpus luteum has undergone internal hemorrhage, it may appear as hyperintense on T1-weighted images.



Vagina
The vagina extends from the uterine cervix to the vulva. The vaginal walls are composed of 3 layers: the mucosa, muscle, and adventitia. The vagina is best imaged on T2-weighted sequences where the mucosa and intraluminal secretions are better seen owing to their hyperintensity; the muscle layer is quite hypointense in comparison ( Fig. 6 ). The sagittal plane best visualizes the vaginal length. Some institutions may insert an inert gel to distend the vagina aiding in the evaluation of vaginal septa. However, this procedure may not be well-tolerated in the pediatric population.

Male
Prostate
The prepubescent prostate is often difficult to see with imaging. Although the differentiation of glandular tissues occurs throughout infancy and childhood, it is not until puberty that the prostate grows in size and the lobar anatomy becomes defined ( Fig. 7 ). The prepubertal prostate is homogeneously isointense to muscle on T1-weighted images and isointense to hyperintense to muscle on T2-weighted images ( Fig. 8 ). After puberty, the imaging characteristics are similar to the adult prostate.


Testes
On T1-weighted images, the normal testis demonstrates a low to intermediate signal intensity including the mediastinum testis. On T2-weighted images, the testis is hyperintense. The surrounding tunica albuginea is hypointense on T1- and T2-weighted images. The epididymis is easier to identify on T2-weighted images owing to its lower signal intensity than the testis. The mediastinum testis is relatively hypointense to the surrounding testis on T2-weighted images but similar in signal intensity to the remainder of the testis on T1-weighted images.
Image interpretation
Congenital Disorders
Müllerian duct anomalies
MDAs refer to the fetal nondevelopment, impaired fusion, or incomplete septal resorption of the Müllerian duct system. MDAs are often associated with infertility, pregnancy complications, and endometriosis. The prevalence of MDA in women with histories of infertility or miscarriage may be as high as 25%. However, the true prevalence in the general population is unknown because many women are asymptomatic.
Embryology
The Müllerian (paramesonephric) ducts give rise to the fallopian tubes, uterus, cervix, and the upper two-thirds of the vagina. For the first 6 weeks of gestation, all fetal genital systems are identical. Paired Müllerian and Wolffian (mesonephric) ducts are initially both present. However, the absence of Müllerian inhibiting substance (MIS), found on the Y chromosome, allows the Müllerian ducts to proliferate after the sixth week while the Wolffian ducts regress. If this process is interrupted early, aplasia or hypoplasia of the uterus, cervix, and upper vagina can occur. The lower one-third of the vagina comes from the urogenital sinus and is separated from the upper vagina by the vaginal plate, which becomes the hymen. Normally, the distal Müllerian ducts reach the urogenital sinus to form the full vagina; failure to do so results in vaginal agenesis. At 7 to 9 weeks of gestation, the Müllerian ducts migrate toward the middle and fuse to form the uterovaginal primordium (uterovaginal canal). Interruption of this process leads to a unicornuate or didelphys uterus. At 9 to 12 weeks of gestation, the uterovaginal septum resorbs; disruption of this process leads to a septate or arcuate uterus. By 12 weeks of gestation, the development of the uterus is complete. Abnormal Wolffian duct development affects normal renal and Müllerian duct development. As such, MDAs are often associated with other congenital abnormalities, especially renal.
Classification system
Although there is controversy regarding the classification and nomenclature of the MDAs, the most widely accepted classification system is by the American Fertility Society, now known as the American Society of Reproductive Medicine, developed in 1998. This system divides the MDAs into 7 classes by major anatomic uterine types with subtypes ( Table 2 ). The American Fertility Society classification system works best for postpubescent uteri because the neonatal and prepubescent uteri may be too small for accurate characterization. This classification system only refers to the uterus and does not include an approach to classify congenital obstructive abnormalities of the vagina or cervix. It also does not allow for classification of the uterus if it shows 2 or more anomalies. Owing to a lack of a universal classification system, detailed descriptions of the anatomy of the uterus, cervix and vagina may be the best approach.
| Class | Uterine Abnormality | Imaging Notes |
|---|---|---|
| Hypoplasia or agenesis of the paired Müllerian ducts | A hypoplastic uterus often will not have the normal trilaminar zonal anatomy; sagittal plane important to assess for degree of vaginal agenesis |
| Agenesis or hypoplasia of 1 of the Müllerian ducts | Unicornuate uterus is banana shaped with a narrow endometrial cavity that tapers at the fundus; always look for endometrium in a rudimentary horn |
| Failure of lateral fusion of the Müllerian ducts | Both horns are normal in size and fully developed with normal zonal anatomy |
| Incomplete fusion of the Müllerian ducts at the level of the body and fundus | The horns of a bicornuate uterus may be smaller than that of a didelphys uterus; normal zonal anatomy |
| Incomplete or no resorption of the uterovaginal septum | Uterine fundus is convex, flat or minimally concave (<10 mm); hypointense septum if fibrous, can be relatively hyperintense on T2-weighted images if muscular |
| Normal variant, near-complete resorption of the uterovaginal septum | Smooth, broad-based fundal impression into the endometrial cavity; outer uterine fundal contour remains convex or flat |
| T-shaped uterine cavity with shortened upper uterus | Not seen in the current pediatric population |
Class I
Class I anomalies refer to hypoplasia or agenesis of the uterus, cervix, or upper two-thirds of the vagina. This anomaly is caused by the failure of the Müllerian ducts to develop. The most common form is the Mayer–Rokitansky–Kuster–Hauser syndrome, which refers to the combined agenesis of the uterus, cervix, and upper portion of the vagina. Because the ovaries are normal, affected patients usually have normal external female genitalia and a normal female karyotype. However, a careful search should always be made for a hypoplastic uterine structure because a rudimentary uterus could still contain an endometrial cavity; obstruction of the endometrial cavity could lead to pain and endometriosis. The hypoplastic uterus demonstrates typical myometrial signal intensity, but often does not have the normal trilaminar zonal anatomy.
Class II
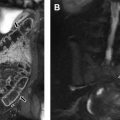
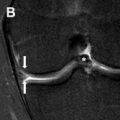
Class II anomalies are referred to as a unicornuate uterus and result from the arrest (total or near total) of 1 Müllerian duct. With 90% of cases of a unicornuate uterus, the arrest is incomplete and a rudimentary horn is present, with or without a functioning endometrium ( Fig. 9 ). MR imaging can demonstrate the unicornuate uterus and identify the presence or absence of a rudimentary horn with or without communication with the contralateral horn. The unicornuate uterus appears as a banana-shaped structure off the midline with a narrow endometrial cavity that tapers at the fundus. Endometrium in a rudimentary horn can often be distinguished by the presence of zonal anatomy. An obstructed rudimentary horn is often filled with hyperintense blood products on T1-weighted images and a patient may present during puberty owing to pain from accumulating fluid.

Class III
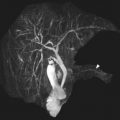
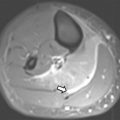
A class III anomaly is the didelphys uterus, which occurs owing to complete nonfusion of both Müllerian ducts. Both horns are usually normal in size and fully developed. Two cervices and upper vaginas are also present. MR imaging demonstrates 2 divergent uterine horns with normal zonal anatomies and normal volumes of the endometrial cavities ( Fig. 10 ). The American Society of Reproductive Medicine states that the uterine cleft between the divergent horns must be deeper than 10 mm; however, this cutoff has limited use in the pediatric population where the entire prepubertal uterus may not reach a length much longer than 1 cm.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree