Neck masses are commonly encountered in the pediatric population, resulting from congenital, neoplastic, inflammatory, and infectious causes. MR imaging may be used for the evaluation and characterization of these lesions. This article discusses a variety of commonly encountered neck masses and lesions in the pediatric population, with an emphasis on up-to-date MR imaging techniques and key diagnostic features. Clear understanding of MR imaging findings of various congenital and acquired neck masses in the pediatric population has a great potential for accurate and timely diagnosis and optimal pediatric patient care.
Key points
- •
Neck masses are commonly encountered in the pediatric population.
- •
In addition to MR imaging findings, patient age and clinical presentation are important to know in order to develop a focused differential diagnosis for a pediatric neck mass.
- •
MR imaging is a valuable noninvasive imaging modality for the evaluation of pediatric neck masses.
- •
MR imaging may be used in conjunction with ultrasound, and at times with CT, to provide complementary information that can aid in diagnosis and management of pediatric neck masses.
Introduction
Neck masses are commonly encountered in the pediatric population, with a wide spectrum of underlying causes, including congenital, infectious, inflammatory, and vascular lesions, as well as benign and malignant neoplasms. Patient age, presentation, and the rapidity of the developing neck mass are important clues guiding the clinician to a focused differential diagnosis. On imaging, lesion features, location, and associated findings are important to help the radiologist establish a reasonable differential diagnosis, if not the diagnosis itself.
Ultrasound remains among the most widely used imaging modalities for the initial evaluation of pediatric neck masses. MR imaging is increasingly used in the pediatric population, offering superior soft tissue contrast for lesion characterization, assessment of lesion extent, and evaluation of the lesion to pertinent surrounding anatomic structures, which are important for diagnosis and, when applicable, pretreatment planning. The MR imaging sequences most commonly used include high-resolution, small field-of-view, thin-slice axial and coronal T1-weighted and T2-weighted images, including contrast-enhanced sequences. Diffusion-weighted imaging and fat-suppressed sequences may be helpful as well. In all cases, the MR imaging sequences should be tailored to the location and the suspected diagnosis.
In this article, the pertinent clinical, epidemiologic, pathologic, and radiologic features of the most commonly encountered pediatric neck masses are discussed, with an emphasis on the current role and use of MR imaging.
Congenital masses
Cystic lesions represent a large percentage of congenital neck masses. These lesions typically result from disrupted fetal formation of structures such as the thyroid gland and thymus, disrupted embryologic development of the branchial apparatus, or may be malformative in situ, resulting in lymphatic malformations (LMs), dermoid or epidermoid cysts, and teratomas. The most common congenital lesion encountered is the thyroglossal duct cyst (TGDC).
Thyroglossal Duct Cyst
The thyroid primordium becomes apparent around the fourth week of fetal life as a median outgrowth from the floor of the primitive pharynx, at the level of the foramen cecum. The developing primordium elongates and descends, passing anterior and then inferoposterior to the developing hyoid bone before continuing down and bifurcating into the left and right lateral anlagen on either side of the median anlage. The gland is connected to its site of origin by the thyroglossal duct up until the seventh gestational week when the thyroid gland reaches its final destination and the duct regresses. In almost half the population, the distal portion of the duct persists as the pyramidal lobe. A TGDC arises when portions of the duct fail to involute.
TGDC is the most common congenital neck mass, accounting for 70% of congenital neck anomalies, and is the second most common benign neck mass (after benign lymphadenopathy). Most affected patients present before age 20 years with a painless palpable mass. Sudden enlargement suggests superimposed inflammation or infection. TGDCs are often located in the midline (75% of cases) or slightly off-midline (25%) in the anterior neck, with an intimate association with the hyoid bone and/or strap muscles in accordance with the embryologic course of the duct. Most TGDCs (80%) are infrahyoid or at the level of the hyoid. In rare cases, a TGDC may occur at the floor of mouth. Thyroid tissue may be found within a TGDC; thus thyroid pathologic conditions, such as goiter or malignancy (usually papillary thyroid carcinoma; 80% of cases) can occur in a TGDC, although they are rarely seen in children.
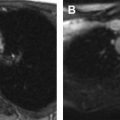
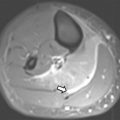
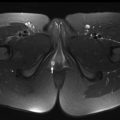
On MR imaging, a TGDC is typically located in the midline or paramidline anterior neck, most often at the level of the hyoid bone or caudal to that within the strap muscles ( Fig. 1 ). It is T1-hypointense, T2-hyperintense, and nonenhancing, reflective of simple fluid content. Less commonly, it may occur within the posterior tongue in the region of the foramen cecum ( Fig. 2 ). T1 isointensity to hyperintensity may be seen with proteinaceous content. Thick irregular rim enhancement has been described in cases of superimposed infection, inflammation, and hemorrhage. If enhancement appears bulky, an internally developing thyroid carcinoma should be considered.


Ectopic thyroid gland tissue may also present as a neck mass along the course of the thyroid primordial path. In rare instances, it may occur off-midline. Although ectopic thyroid tissue is typically asymptomatic, patients may present with localized swelling, dysphagia, stridor, or dysphonia. On MR imaging, ectopic thyroid tissue appears mildly T2-hyperintense with avid enhancement.
Surgical treatment of TGDC requires complete resection of the lesion and tract up to the hyoid (Sistrunk procedure) to prevent recurrence.
Branchial Anomalies
The branchial apparatus consists of paired arches, each with its corresponding pouch, cleft (or groove), and membrane, giving rise to structures of the face and neck. Branchial anomalies arise from either vestigial remnants in incompletely obliterated regions of the branchial apparatus or proliferation of buried cell rests, resulting in a cyst (closed off internally and externally), sinus (open either internally to the pharyngeal lumen or externally to the skin), or fistula (open internally and externally). A branchial anomaly is numbered according to its pouch or cleft of origin. Of note, the fifth and sixth arches are rudimentary or noncontributory to anatomic structures, and fifth and sixth branchial anomalies have not been reported. Complete surgical excision is the mainstay of treatment common to all 4 types of branchial cleft cysts (BCCs).
First branchial anomaly
The first branchial cleft gives rise to the external auditory canal and the first branchial membrane gives rise to the tympanic membrane. As such, first branchial anomalies have a close relationship with the external auditory canal but can be found inferior to that, within or around the parotid gland down to the level of the hyoid. Cysts account for two-thirds of first branchial anomalies, with the remainder divided between fistulae and sinuses. Overall, first branchial anomalies are relatively uncommon, accounting for about 8% of all branchial anomalies. In 1972, Work classified first branchial anomalies into two types. Work type I lesions are of ectodermal origin, and considered duplications of the membranous auditory canal. Work type II lesions contain both ectoderm and mesoderm, and are considered duplication anomalies of the membranous external auditory canal and pinna.
Clinically, affected pediatric patients present with recurrent infection or abscess or inflammation around the ear or in the parotid or periparotid region down to the angle of the mandible, either involving a cyst or along a sinus or fistula. Otorrhea can occur if the lesion drains into the external auditory canal.
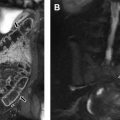
On MR imaging, first BCC appears as a T1-hypointense, T2-hyperintense, cystic lesion superficial to or within the parotid gland ( Fig. 3 ), or inferior to the parotid gland down to the level of the hyoid ( Fig. 4 ). Differential diagnosis includes a sialocele if it is intraparotid, a cystic pilomatrixoma if periparotid against the skin, suppurative or necrotic adenopathy (including atypical mycobacterial infection), abscess, lymphatic malformation, and dermoid cyst. If a sinus tract draining into the external auditory canal can be identified on thin-slice high-resolution T2-weighted MR imaging, a first branchial anomaly is strongly suggested.


Second branchial anomaly
Second branchial anomalies are the most common, accounting for greater than 92% of all branchial anomalies. Cysts are much more common than fistulae or sinuses. Bailey classified second BCCs into 4 types based on location. Bailey type II second BCC is the most common type, located at the level of the angle of the mandible posterior to the submandibular gland, anterior to the sternocleidomastoid muscle, and superficial to the carotid sheath. Clinically, a second BCC presents as a painless, fluctuant mass in the lateral upper neck. The mass may suddenly increase in size and be tender if secondarily infected.
On MR imaging, a second BCC is well-circumscribed, with a uniform, thin peripheral wall. Most are located at the anteromedial border of the sternocleidomastoid muscle, lateral to the carotid sheath, and posterior to the submandibular gland. These lesions are T1-hypointense and T2-hyperintense ( Fig. 5 ). The beak sign refers to a pointed margin of the cyst insinuating between the internal and external carotid arteries. An uninfected cyst shows minimal rim enhancement; however, in the setting of inflammation and/or infection, more nodular and thickened wall enhancement may be noted.

Third or fourth branchial anomaly
Third and fourth branchial anomalies are difficult to distinguish from each other radiologically, and almost all of both occur on the left side of the neck. Third or fourth BCCs are extremely rare, with only a few cases reported in the literature. When seen, a third BCC, following embryologic route, should be found posterior to the carotid artery and posterior to the sternocleidomastoid muscle, at a level caudal to a second BCC. A fourth BCC would occur even more caudally, adjacent to the thyroid gland.
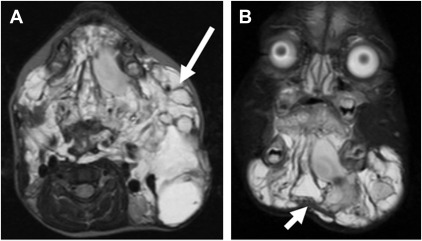
On MR imaging, as with first and second BCCs, a third or fourth BCC appears as a unilocular T1-hypointense, T2-hyperintense lesion within the region of the posterior neck, abutting the posterior margin of the sternocleidomastoid muscle. Often, there is a thin rim of peripheral enhancement; however, in the setting of superinfection, thickened wall enhancement and heterogeneous internal contents may be seen ( Fig. 5 ). These lesions may have an opening at the upper pyriform sinus (third BCC) or the apex of the pyriform sinus (fourth BCC), and/or a cutaneous opening in the supraclavicular area anterior to carotid artery.
However, more commonly, patients with a third or fourth branchial anomaly present with the sequelae of a sinus tract or fistula that opens into the pyriform sinus (pyriform sinus fistula), manifesting as recurrent neck swelling and inflammation related to episodes of thyroid and/or perithyroidal infections from seeding of the tract and surrounding soft tissues by bacteria in the pharynx. On MR imaging, inflammatory changes, including edema and fat infiltration, are seen involving the thyroid parenchyma and perithyroidal tissues; a fluid collection indicates a phlegmon or abscess ( Fig. 6 ). Following resolution of acute inflammatory changes, a fluoroscopic pharyngogram may be performed to assess for a potential fistulous tract extending from the pyriform sinus caudally within the neck tissues, although false-negative results are not uncommon and the tract may only be evident on surgical exploration.

Thymic Cysts
The thymus arises from the third branchial (pharyngeal) pouch. Rarely, a minor contribution comes from the fourth pouch. Thymic primordia descend from an outpouching down a tract, termed the thymopharyngeal duct, on either side of the neck. The duct on each side courses caudally, lateral to the ipsilateral pyriform sinus, lateral and inferior to the ipsilateral thyroid lobe, and the 2 ducts then merge inferior to the thyroid gland to continue descent to the mediastinum. At the sixth to seventh weeks of gestation, the thymopharyngeal duct involutes. If the duct fails to involute completely, a thymopharyngeal duct cyst and/or thymic cyst may occur.
Cervical thymic cysts are very uncommon lesions, often detected within the first decade of life. These lesions may be found anywhere along the path of the thymopharyngeal duct, which courses immediately adjacent to the carotid sheath extending from the level of the angle of the mandible to the thoracic inlet and may extend into the mediastinum. A fibrous strand may connect a thymic cyst with the thyroid gland. Cervical thymic cysts are more common on the left-side.
Clinically, thymic cysts are often asymptomatic but may demonstrate slow progressive enlargement. Rarely, symptoms include hoarseness, dysphagia, stridor, and respiratory distress in newborns. Sudden enlargement may be seen with Valsalva maneuvers (likely related to increased intrathoracic pressure), hemorrhage, or viral infection. Treatment is with surgical resection but approximately 2% recur.
On MR imaging, thymic cysts appear as a large, unilocular, T1-hypointense, and T2-hyperintense cystic lesions with rim enhancement. It is often oriented parallel to the long axis of the sternocleidomastoid muscle ( Fig. 7 ).

If there is incomplete descent of thymic primordia, or if there is sequestration of thymic tissue along the duct, ectopic thymus results. Locations where this may be seen are similar to those for thymic cysts. However, on imaging, ectopic thymus appears as a solid mass with pliable margins.
Dermoid Cyst
Dermoid cysts are circumscribed, encapsulated congenital lesions lined by squamous epithelium, containing skin appendages such as sebaceous gland and hair follicles. They typically manifest during the second to third decades of life and have no gender predilection. A minority (∼7%) of dermoid cysts occur in the head and neck, with the lateral aspect of the eye and the floor of mouth being common locations. In the neck, a dermoid often appear as a midline, suprahyoid, slow-growing mass that does not move with tongue protrusion. Uncommonly (5%), a dermoid cyst may undergo malignant degeneration into squamous cell carcinoma.
On MR imaging, a dermoid cyst is T2-hyperintense, with variable T1 signal related to the presence of lipid ( Fig. 8 ). Determining the relationship of a dermoid to the mylohyoid muscle in the floor of the mouth is important for selecting an appropriate surgical approach. Those located superior to the mylohyoid muscle can be removed via an intraoral approach, whereas those inferior to this muscle can be removed via an external submandibular approach.

Teratoma
Cervical teratomas are unusual neck masses that usually occur in newborns or young infants; the estimated occurrence in neonates is approximately 1:20,000 to 40,000 live births. Head and neck involvement accounts for 2% of teratomas, with most occurring in or adjacent to the thyroid gland. All 3 germinal layers are present and lesions may contain fat, teeth, and soft-tissue elements. Most teratomas are diagnosed at birth, although some may be diagnosed in utero. Prognosis is good if there is no respiratory compromise. Antenatal complications include development of hydrops fetalis if there is significant vascular shunting in utero. Malignant transformation, though rare, has been described.
On MR imaging, these lesions often appear multiloculated and may mimic lymphatic malformations. T1 and T2 signal intensity and enhancement are highly variable and depend on lesion composition and complexity ( Fig. 9 ); there are usually cystic with varying amounts of fatty and soft tissue components. Calcifications can be seen in approximately half of cases. The differential diagnosis includes LM and angiomyolipoma.

Treatment entails surgical resection, which may be technically demanding if the lesion is closely related to adjacent vital structures. Recurrence following surgical excision is uncommon.
Vascular malformations
More than half of the vascular lesions in the pediatric population are found in the head and neck, and may be identified from infancy through late childhood. Vascular lesions can be divided into 2 categories: tumors and malformations. Vascular tumors are true neoplasms and include infantile hemangioma, congenital hemangioma, and the much less common kaposiform hemangioendothelioma. Management options for vascular tumors include watchful waiting, oral medications, and surgical intervention. Vascular malformations are not neoplasms and are further subdivided into low-flow and high-flow lesions. Low-flow lesions include venous and lymphatic malformations and combined (venous and lymphatic) malformations. High-flow lesions include arteriovenous malformations (AVMs). The treatment of vascular malformations includes endovascular and/or surgical obliteration.
Hemangiomas (Vascular Neoplasm)
Congenital hemangiomas
Rapidly involuting congenital hemangioma (RICH) is a benign, vascular tumor with increased endothelial turnover, leading to enlargement in childhood. It is the most common tumor of infancy, occurring in 12% to 23% of preterm infants. It is slightly more frequent in infants with low birthweight and has a 3:1 female predilection. Following a period of characteristic growth, RICHs often rapidly involute and do not require surgical treatment. These lesions can be seen in the setting of cutaneous hemangioma-vascular complex syndrome, a phakomatosis comprising posterior fossa malformations, hemangiomas, arterial anomalies, aortic coarctation, cardiac anomalies, eye anomalies, and sternal clefting and/or supraumbilical raphe (PHACES).
Noninvoluting congenital hemangioma (NICH) is much less common and has a male to female predilection of 3:2. A main difference between RICH and NICH lesions is that NICH never regresses but instead grows in proportion with the child, and may require eventual surgical excision if the lesion leads to symptomatic or cosmetic sequelae.
On MR imaging, RICH and NICH appear similar and are of intermediate T1 intensity, elevated T2 intensity, with prominent internal flow voids and avid enhancement ( Fig. 10 ). Evaluation of additional structures in the brain, orbits, vessels, and mediastinum is important to exclude PHACES syndrome ( Figs. 11 and 12 ).



Infantile hemangioma
Infantile hemangioma is a benign vascular tumor of infancy that, unlike congenital hemangioma, is not present at birth. It usually presents within the first few weeks of life, and is characterized by early rapid proliferation, followed by a growth plateau by the end of the first year of life, and eventual spontaneous slow involution that may continue through age 5 to 10 years. Greater than half occur in the head and neck, and they may be cutaneous or subcutaneous in location.
MR imaging cannot differentiate between infantile and congenital hemangiomas because both appear mass-like and exhibit prominent internal flow voids and avid enhancement. The diagnostic distinction relies on clinical history: whether the lesion was present at birth (congenital) or not (infantile). MR imaging is, however, useful to help distinguish hemangiomas from other high-flow lesions, such as AVM, and in defining the extent of the lesion and involvement of adjacent structures.
Low-Flow Vascular Malformations
Lymphatic malformation
Lymphatic malformations (LMs) constitute approximately 5% of all benign masses in infancy and childhood. Most are detected by age 2 years, during the time of greatest lymphatic growth. No gender predilection has been reported. These lesions are typically asymptomatic but may manifest as painless, soft, or semifirm masses in the neck. A large LM can potentially compromise the airway by extrinsic pressure. Conventionally, LMs are slow-growing but may demonstrate a rapid increase in size secondary to hemorrhage, trauma, or infection when large quantities of lymphatic fluid are produced. Additionally, facial nerve paralysis, dysphagia, and feeding problems have been reported, depending on lesion location.
Histopathologically, LMs may be macrocystic or microcystic. Macrocystic LMs are more common and thought to result from the sequestration of embryonic lymphatic channels; this has the characteristic infiltrative and trans-spatial appearance on imaging. Microcystic LM may be dominated by innumerable enhancing septations between tiny cysts.
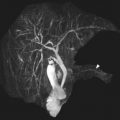
On MR imaging, LMs have pathognomonic features, appearing well-circumscribed, sharply marginated, trans-spatial, with internal cystic spaces separated by thin enhancing septations. With internal hemorrhage, the fluid and blood products separate from each other based on density, resulting in fluid layers of varying signal characteristics ( Fig. 13 ). Superimposed infection may alter the T1 signal intensity and enhancement characteristics.
Venous malformations
Venous malformations (VMs) are benign, slow-flow malformative lesions containing abnormally dilated and tortuous veins and abnormal endothelial lining. VMs constitute up to 40% of all diagnosed vascular anomalies and have no gender predilection. VMs may enlarge around puberty, requiring surgical and/or endovascular intervention. Some VMs involve adjacent soft tissues and bone, leading to overgrowth, and can lead to significant facial disfigurement and/or disability.
Clinically, a VM appears bluish and fluctuant, and may enlarge with Valsalva maneuvers. VMs are usually located at or near the cutaneous surface. Vascular bruit and thrill are not appreciated because these are low-flow lesions. Affected pediatric patients may experience chronic pain related to venous stasis, congestion, and thrombosis.
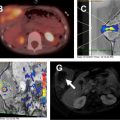
On MR imaging, VMs demonstrate T2 hyperintensity ( Fig. 14 ). Small internal signal voids are suggestive of calcifications, although these are more conspicuous and definitively assessed on computed tomography (CT) ( Fig. 14 C and D). Enhancement is variable. VMs may have a trans-spatial appearance related to their predilection to extend along fascial planes.