Incidentally detected adnexal masses are common, and the overwhelming majority of them are benign. As many of these adnexal masses are considered indeterminate at CT or US, a large number of benign oophorectomies occur. Of the malignant adnexal masses, high-grade primary ovarian neoplasms with fast doubling times and early dissemination are the most common. Due to their aggressive behavior, diagnosis of malignancy by interval growth on surveillance imaging represents an undesirable option. Immediate MR characterization allows for a decreased rate of benign oophorectomies and expedited triage of patients to definitive treatment when malignancy is suspected.
Key points
- •
Incidental adnexal masses considered indeterminate for malignancy are commonly seen on computed tomography (CT) scan and ultrasound (US). Most of these prove to be benign.
- •
Ovarian cancer grows rapidly; thus, adnexal masses that cannot be definitively characterized as benign on imaging are resected to avoid a clinically significant delay in cancer diagnosis.
- •
Pelvic MR imaging increases the specificity of the imaging evaluation and enables confident diagnosis of many benign lesions.
- •
Incorporation of MR imaging into imaging evaluation of adnexal masses allows for decreased resection rates of benign lesions and aids in treatment planning for minimally invasive interventions.
Introduction
Incidentally detected adnexal lesions are common, identified in approximately 4% to 5% of women undergoing computed tomography (CT) scan and in 9% to 10% of women undergoing ultrasound (US). Although a source of anxiety for patients, referring clinicians, and radiologists alike, these lesions, or the overwhelming majority, are benign. Nevertheless, concern over incidentally detected adnexal lesions results in high rates of benign oophorectomies. The frequency of malignancy in premenopausal women undergoing surgical resection of an adnexal mass is well below 10%, and that for postmenopausal women is less than 15%. In the United States, between 5% and 10% of women will undergo oophorectomy for a benign adnexal lesion. These numbers are not trivial given the associated surgical morbidity and downstream health consequences of early surgical menopause, which include infertility and increased prevalence of coronary artery disease, cardiovascular death, dementia, and Parkinsons disease.
The incidence of ovarian cancer is known to increase with age, and the lifetime risk is approximately 1.3%. Despite this, most adnexal masses identified even in elderly women are benign. One case series examining the postmortem adnexa of women who had died of nongynecologic causes found 15.4% (36/234) had adnexal cysts, only 1 of which was found to be malignant (serous borderline cancer). Rapid growth rate of high-grade ovarian cancers means that the choice to defer definitive diagnosis to follow-up imaging may result in a clinically significant spread of cancer. Screening trials have shown that most ovarian cancers show rapid progression from early-stage sonographically detectable lesion to extraovarian spread with short lag times of weeks to months. In 1 trial, all 10 of the ovarian cancers detected were at stage III or IV, having developed within the 6-month interval between screening examinations. A recent study examining 118 incidentally detected adnexal lesions in 2869 women consecutively screened with CT colonography found that all incidentally detected lesions were benign. Ironically, during the follow-up period, 4 cases of ovarian cancer were diagnosed in the 2751 women not found to have adnexal lesions at screening CT colonography.
The differential diagnosis of adnexal masses is broad ( Box 1 ) and includes ovarian and extraovarian abnormalities, which are both benign and malignant. MR imaging has been shown to be the most accurate modality in adnexal mass characterization, and many of the benign adnexal lesions considered indeterminate at CT and US (eg, physiologic cysts, hemorrhagic cysts, cystadenomas, mature cystic teratomas, endometriomas, hydrosalpinges, and paratubal cysts) may be confidently diagnosed as benign with MR imaging. This accuracy is due to superior soft tissue assessment and lesion localization. The diagnostic accuracy of MR imaging versus CT for diagnosis of malignancy after US detection of an indeterminate adnexal mass may be seen in Table 1 . Confident diagnosis of adnexal lesion as benign at imaging allows for an overall decrease in resection rates for benign abnormality and appropriate triaging of patients to minimally invasive and/or fertility-sparing procedures. Conversely, patients with indeterminate adnexal lesions that remain indeterminate or exhibit malignant features at MR imaging may be rapidly triaged to appropriate surgical management.
Ovarian benign (common)
Epithelial
Physiologic cyst
Endometrioma
Cystadenoma
Nonepithelial
Mature cystic teratoma (dermoid)
Fibroma
Ovarian borderline and malignant (rare)
Epithelial
Serous carcinoma
Mucinous carcinoma
Clear cell carcinoma
Endometrioid carcinoma
Brenner or transitional cell carcinoma
Nonepithelial
Germ cell, for example, dysgerminoma
Sex cord stromal, for example, granulosa cell, Seroli-Leydig cell
Metastases: uterine, gastrointestinal, pancreatic, & breast primaries
Rare histologies, for example, carcinosarcoma, primitive neuroectodermal tumor
Extraovarian benign (common)
Uterus
Fibroid
Obstructed horn
Adenomyoma
Broad ligament
Fallopian tube: hydrosalpinx, hematosalpinx, pyosalpinx
Paratubal (paraovarian) cyst
Nongynecologic organs
Peritoneal inclusion cyst
Bowel: appendiceal mucocele, duplication cyst
Lymphatics–lymphocele, lymphangioma
Fibromatosis or desmoid tumor
Nerve sheath tumor, for example, schwannoma
Extraovarian malignant (very rare)
Colorectal carcinoma
Primary peritoneal carcinoma
Gastrointestinal stromal tumor
Malignant transformation of endometriosis
Lymphoma
Mesothelioma
Note: List ordered from higher to lower incidence within each subgroup.
| Modality | Sensitivity % | Specificity % |
|---|---|---|
| CT with contrast | 81 (73, 86) | 87 (81–94) |
| MR without contrast | 76 (70, 82) | 97 (95, 98) |
| MR with contrast | 81 (77, 84) | 98 (97, 99) |
Introduction
Incidentally detected adnexal lesions are common, identified in approximately 4% to 5% of women undergoing computed tomography (CT) scan and in 9% to 10% of women undergoing ultrasound (US). Although a source of anxiety for patients, referring clinicians, and radiologists alike, these lesions, or the overwhelming majority, are benign. Nevertheless, concern over incidentally detected adnexal lesions results in high rates of benign oophorectomies. The frequency of malignancy in premenopausal women undergoing surgical resection of an adnexal mass is well below 10%, and that for postmenopausal women is less than 15%. In the United States, between 5% and 10% of women will undergo oophorectomy for a benign adnexal lesion. These numbers are not trivial given the associated surgical morbidity and downstream health consequences of early surgical menopause, which include infertility and increased prevalence of coronary artery disease, cardiovascular death, dementia, and Parkinsons disease.
The incidence of ovarian cancer is known to increase with age, and the lifetime risk is approximately 1.3%. Despite this, most adnexal masses identified even in elderly women are benign. One case series examining the postmortem adnexa of women who had died of nongynecologic causes found 15.4% (36/234) had adnexal cysts, only 1 of which was found to be malignant (serous borderline cancer). Rapid growth rate of high-grade ovarian cancers means that the choice to defer definitive diagnosis to follow-up imaging may result in a clinically significant spread of cancer. Screening trials have shown that most ovarian cancers show rapid progression from early-stage sonographically detectable lesion to extraovarian spread with short lag times of weeks to months. In 1 trial, all 10 of the ovarian cancers detected were at stage III or IV, having developed within the 6-month interval between screening examinations. A recent study examining 118 incidentally detected adnexal lesions in 2869 women consecutively screened with CT colonography found that all incidentally detected lesions were benign. Ironically, during the follow-up period, 4 cases of ovarian cancer were diagnosed in the 2751 women not found to have adnexal lesions at screening CT colonography.
The differential diagnosis of adnexal masses is broad ( Box 1 ) and includes ovarian and extraovarian abnormalities, which are both benign and malignant. MR imaging has been shown to be the most accurate modality in adnexal mass characterization, and many of the benign adnexal lesions considered indeterminate at CT and US (eg, physiologic cysts, hemorrhagic cysts, cystadenomas, mature cystic teratomas, endometriomas, hydrosalpinges, and paratubal cysts) may be confidently diagnosed as benign with MR imaging. This accuracy is due to superior soft tissue assessment and lesion localization. The diagnostic accuracy of MR imaging versus CT for diagnosis of malignancy after US detection of an indeterminate adnexal mass may be seen in Table 1 . Confident diagnosis of adnexal lesion as benign at imaging allows for an overall decrease in resection rates for benign abnormality and appropriate triaging of patients to minimally invasive and/or fertility-sparing procedures. Conversely, patients with indeterminate adnexal lesions that remain indeterminate or exhibit malignant features at MR imaging may be rapidly triaged to appropriate surgical management.
Ovarian benign (common)
Epithelial
Physiologic cyst
Endometrioma
Cystadenoma
Nonepithelial
Mature cystic teratoma (dermoid)
Fibroma
Ovarian borderline and malignant (rare)
Epithelial
Serous carcinoma
Mucinous carcinoma
Clear cell carcinoma
Endometrioid carcinoma
Brenner or transitional cell carcinoma
Nonepithelial
Germ cell, for example, dysgerminoma
Sex cord stromal, for example, granulosa cell, Seroli-Leydig cell
Metastases: uterine, gastrointestinal, pancreatic, & breast primaries
Rare histologies, for example, carcinosarcoma, primitive neuroectodermal tumor
Extraovarian benign (common)
Uterus
Fibroid
Obstructed horn
Adenomyoma
Broad ligament
Fallopian tube: hydrosalpinx, hematosalpinx, pyosalpinx
Paratubal (paraovarian) cyst
Nongynecologic organs
Peritoneal inclusion cyst
Bowel: appendiceal mucocele, duplication cyst
Lymphatics–lymphocele, lymphangioma
Fibromatosis or desmoid tumor
Nerve sheath tumor, for example, schwannoma
Extraovarian malignant (very rare)
Colorectal carcinoma
Primary peritoneal carcinoma
Gastrointestinal stromal tumor
Malignant transformation of endometriosis
Lymphoma
Mesothelioma
Note: List ordered from higher to lower incidence within each subgroup.
| Modality | Sensitivity % | Specificity % |
|---|---|---|
| CT with contrast | 81 (73, 86) | 87 (81–94) |
| MR without contrast | 76 (70, 82) | 97 (95, 98) |
| MR with contrast | 81 (77, 84) | 98 (97, 99) |
Anatomy
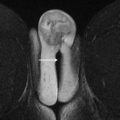
The uterine adnexum ( Fig. 1 ) comprises the ovary, the fallopian tube, the broad ligament, the ovarian ligament, the round ligament, and the infundibular pelvic ligament. The ovarian ligament connects the ovaries to the uterine fundus and then continues into the inguinal canal as the round ligament. The infundibular ligament, also referred to as the suspensory ligament of the ovary, is the lateral extension of the broad ligament, which anchors the ovary to the pelvic sidewall. The broad ligament is a 2-layered peritoneal fold that connects the uterus to the pelvic sidewall and the pelvic floor. Its contents include the ovary, the ovarian artery, vein, and lymphatics laterally, the uterine artery, vein, and lymphatics medially, the fallopian tube, and the ovarian and round ligaments. Ovarian arteries originate from the abdominal aorta below the renal artery origins. The right ovarian vein generally drains into the inferior vena cava, whereas the left ovarian vein drains into the left renal vein.
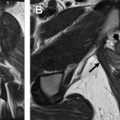
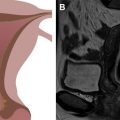
The normal ovaries as well as the broad and round ligaments are typically well seen at pelvic MR imaging. Identifying these structures is a crucial component to accurate pelvic MR imaging interpretation because the identification of normal ovaries on imaging substantially informs the differential diagnosis of an adnexal mass. The ovaries are located at the junction of the broad and round ligaments, and these structures may be used as landmarks for ovarian identification. Normal ovaries in a premenopausal woman ( Fig. 2 ) will have multiple nonenhancing cysts (follicles) containing homogeneously hyperintense fluid within a stroma that is intermediate in signal on T2-weighted imaging. In a postmenopausal woman, follicles may be less apparent or absent. On postcontrast T1-weighted imaging, the follicular fluid is nonenhancing, and the normal ovarian stroma is relatively hypoenhancing with respect to the myometrium. Using the ligaments as anatomic landmarks is particularly important when identifying postmenopausal ovaries that are small (usually <2 cm), lack follicles ( Fig. 3 ), and can sometimes be mistaken for lymph nodes. The broad ligament may be traced laterally from the uterus, and the round ligament may be traced medially from the inguinal canal. Alternatively, on the postcontrast T1-weighted sequences, the gonadal veins may be traced inferiorly, the left from the left renal vein and the right from the inferior vena cava, to the points where they drain the ovary.
Imaging protocol
MR imaging of the female pelvis for characterization of adnexal masses is best performed with a small field of view and a phased-array surface coil to maximize soft tissue contrast and signal-to-noise. Multiplanar fast spin-echo T2 (FSE T2)-weighted imaging in sagittal, axial, and coronal planes comprises the backbone of pelvic imaging. The high soft tissue contrast afforded by these sequences allows for both anatomic localization and tissue characterization (see Fig. 2 A, B). These sequences are typically the most helpful for localization of adnexal lesions as either ovarian or extraovarian. FSE T2-weighted imaging is also helpful in determining whether the lesion is a unilocular cyst, mixed solid and cystic, or homogeneously solid. T1-weighted imaging without and with fat saturation allows for detection of adnexal lesions containing macroscopic fat. The fat-saturated T1-weighted imaging is used to identify ovarian endometriomas, extraovarian endometriosis, and other lesions with blood degradation products, such as hemorrhagic cysts.
Contrast-enhanced T1-weighted MR imaging with fat saturation is necessary to obtain optimum sensitivity and specificity in adnexal mass characterization. The authors obtain dynamic (multiphasic) postcontrast imaging in the sagittal plane, primarily for assessment of the lesion relative to the uterine myometrium versus endometrium. Subtraction imaging is helpful in confirming the presence or absence of soft tissue enhancement, particularly when assessing tissue that contains natively high T1-weighted signal such as an endometrioma. Solid enhancing tissue within an ovarian lesion raises suspicion for malignancy; however, soft tissue enhancement is not pathognomonic for malignancy because benign neoplasms may have enhancing soft tissue as well. Although some institutions obtain high-temporal-resolution true dynamic contrast-enhanced images with washin and washout curves, the benefit of this has not yet been shown. Recent studies comparing the kinetics of contrast enhancement between benign and malignant lesions have suggested that lesions exhibiting rapid, robust enhancement with washout have a higher likelihood of malignancy.
Diffusion-weighted imaging (DWI) has traditionally been used for lesion detection in body imaging. However, there is increasing evidence supporting its use in lesion characterization, with qualitative assessment affording more accuracy than isolated measurement of apparent diffusion coefficient (ADC) value. When evaluating an ovarian lesion for restricted diffusion, internal reference may be made to the myometrium and/or small bowel wall. Assessment for restricted diffusion requires concurrent evaluation of both DWI and ADC maps. A lesion is considered to demonstrate restricted diffusion if it is hyperintense on DWI and hypointense or isointense on the ADC map. As both benign and malignant ovarian lesions may exhibit restricted diffusion, the presence of restricted diffusion in and of itself does not definitively characterize a lesion as malignant. For instance, both mature cystic teratomas and high-grade serous carcinomas ( Fig. 4 A–D ) typically show regions of diffusion restriction. However, it is very rare for a malignant ovarian lesion not to exhibit restricted diffusion; therefore, lack of diffusion restriction in the solid components of an ovarian lesion may be used as a reliable marker of benignity (see Fig. 4 E–H). The important MR pulse sequences for adnexal mass characterization are summarized in Table 2 .
| MR Pulse Sequence | Relevant Diagnostic Features |
|---|---|
| Multiplanar FSE T2-weighted imaging (sagittal, axial, coronal) |
|
| T1-weighted imaging without and with fat saturation |
|
| Precontrast- and dynamic contrast-enhanced (eg, 20 s, 60 s, and 3 min after bolus) T1-weighted imaging |
|
| Subtraction imaging |
|
| Diffusion-weighted imaging |
|
Imaging in Pregnancy
In the setting of pregnancy, pelvic MR imaging, with its high soft tissue contrast, wide field of view, and lack of ionizing radiation, is recommended to characterize an incidentally detected adnexal mass. MR imaging has the capacity to noninvasively and accurately diagnose a lesion as benign, obviating longitudinal imaging follow-up or intervention. Although hypothetical risks of heat deposition and acoustic injury have been raised at high field strengths, no adverse fetal effects of MR have been documented in the past 3 decades of imaging in pregnancy. Nevertheless, examination performance should adhere to safety guidelines published by imaging societies. Some institutions prefer to limit scanning of pregnant patients to 1.5 T because experience with 3-T scanning to date is limited. Because no fetal harm has been documented, scanning can be performed any time during pregnancy as clinically indicated. However, contrast administration should be avoided because gadolinium-based contrast agents readily pass through the placental barrier into the fetal circulation.
Standardized reporting system: ADNEX MR
In 2013, the ADNEX MR SCORING system was created in an effort to standardize radiologists’ reporting of adnexal lesions. Lesions are assigned a score from 1 to 5 based on MR imaging features, which include morphologic appearance, T1 and T2 signal, and enhancement features, including kinetics on high-temporal-resolution dynamic contrast-enhanced sequences, and diffusion characteristics. An ADNEX MR score of 1 denotes no mass present; 2 denotes a benign mass; 3 denotes a probably benign mass; 4 denotes a mass that is indeterminate for malignancy; and 5 denotes a mass that is probably malignant. In the validation study, lesions assigned an ADNEX score of 4 or higher were associated with malignancy with 93.5% sensitivity and 96.6% specificity, and no malignant masses were assigned a score of 1 or 2. Readers in the study exhibited high interobserver agreement (κ = 0.888) in scoring. Aside from standardizing the reporting of adnexal masses, the scoring system also provides a framework for providing recommendations to guide clinical care. Patients assigned an ADNEX MR score of 4 or 5 should be referred for oncologic evaluation, whereas patients assigned ADNEX MR scores of 1 or 2 need no further management or follow-up for their ovarian finding. Patients assigned an ADNEX MR score of 3 may be followed at the discretion of the radiologist and referring clinician.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree