MR imaging-guided focal therapy is a viable treatment option for patients with localized prostate cancer. After the identification of a malignant focus in the prostate gland on multiparametric MR imaging, treatment can be directed in a precise fashion to the area of interest. The goal of focal therapy is to eradicate prostate cancer while minimizing complications that can affect quality of life. Currently, the most commonly used methods of focal treatment of prostate cancer are cryotherapy, high-intensity focused ultrasound, and laser ablation.
Key points
- •
A greater number of small-volume, low-risk, and intermediate-risk prostate cancers are being detected.
- •
Multiparametric MR imaging serves as a valuable method of assessing the prostate gland for cancer in patients with high clinical suspicion of malignancy.
- •
Once detected, a suspected cancerous lesion in the prostate gland can be subsequently targeted for focal therapy using MR imaging for guidance.
- •
Early data relating to the use of MR imaging–guided focal therapies, including cryotherapy, high-intensity focused ultrasound, and focal laser ablation, have been promising.
Background
Prostate cancer is a major cause of death among men, preceded only by lung cancer in the United States. There has been an increase in the number of prostate cancer cases, localized and low-grade tumors in particular, leading to an interest in the development of alternative treatment methods with fewer complications. The growing number of cases of prostate cancer diagnosed can be attributed in part to a greater reliance on prostate-specific antigen (PSA) as a harbinger of malignancy as well as the adoption of an overall lower clinical threshold for the performance of prostate tissue sampling. Although the US Preventive Services Task Force (USPSTF) advised against the use of PSA as a screening mechanism for certain patients, that is, men of 70 years of age or older, the USPSTF recently updated stance recommends that clinicians conduct periodic checks of serum PSA levels in patients between the ages of 55 and 69. Elevated PSA levels and abnormal digital rectal examinations represent the 2 major criteria currently used in the determination of a patient’s need for prostate biopsy.
Diagnosis and treatment of prostate cancer
The traditional method of acquiring tissue samples of the prostate in a patient suspected of having cancer is to pursue a 12-core biopsy of the gland using a transrectal sonographic approach. This conventional transrectal ultrasound (TRUS)-guided biopsy, however, has several disadvantages. Among these is the failure of a TRUS biopsy to consistently reach the apex of the prostate or the anterior aspect of the gland. This leads to undersampling of these areas and missing anterior and apical cancers. In addition, there may be difficulty in the reliable detection of prostate cancer using sonography. For these reasons, it is not entirely surprising that the rate of false-negative results after a TRUS-guided biopsy is high: up to 47% of patients may have an undetected prostate cancer after sampling. Another problem with random biopsy is the unreliable Gleason score yielded by these biopsies. In approximately 30% of cases, the Gleason score obtained on random biopsy is upgraded on repeat tissue sampling or after prostatectomy.
A more reliable method of detecting and diagnosing prostate cancer is multiparametric MR imaging. Prostate cancer has characteristic MR imaging features, including hypointense signal on T2-weighted imaging, low apparent diffusion coefficient (ADC) signal (restricted diffusion), and early arterial enhancement with subsequent washout on dynamic contrast-enhanced imaging. MR imaging has been increasingly used for identification of targets for biopsy, and targeted biopsy is rapidly emerging as an alternative and more superior diagnostic paradigm. Depending on the experience level of the radiologist reading the MR imaging examination, the accuracy of detection of prostate cancer can range between 70% and 90%.
Once a suspicious site is identified on multiparametric MR imaging, MR imaging can be used either directly or indirectly to guide future biopsy of the prostate gland. With the direct method, tissue sampling is done in-bore while the patient is positioned within the scanner. When MR imaging is used in an indirect manner to perform a biopsy of the prostate gland, it is most often achieved through the use of an MR imaging/ultrasound fusion computer platform. In this scenario, the patient’s earlier diagnostic MR images are able to be fused with real-time sonographic images and serve to guide the trajectory of the core biopsy needle during the procedure. The targeted biopsy paradigm has allowed for a more reliable and accurate mapping of cancer within the prostate gland and improved characterization of cancer aggressiveness at the time of diagnosis.
There are several therapy options currently available to patients diagnosed with prostate cancer, including whole-gland treatment, active surveillance, and, the latest approach, focal therapy. The primary aim of focal therapy is to selectively direct treatment to the index or largest in size cancerous prostatic lesion, while in the process avoiding inadvertent damage to critical locoregional anatomic structures. Among these are the neurovascular bundles, the adjacent urethra and urethral sphincter, the urinary bladder, and the rectum. Focally directed and less-invasive treatment approaches are advantageous, particularly in comparison to whole-gland treatment options, such as a radical prostatectomy, where a greater incidence of post-prostatectomy complications, such as impotence and urinary continence, has been encountered. In addition to clinical factors and patient and physician preference, other criteria used in the determination of whether an individual with localized prostate cancer is a satisfactory candidate for focal treatment are Gleason score of 6 or 7, a PSA level measuring less than 15 ng/mL, and a clinical stage of T1c to T2a, as defined by an international consensus group.
Pros and cons of focal therapy
Focal therapy is an established paradigm in the treatment of several different cancers, including breast, kidney, thyroid, colon, lung, and liver. Its main advantage in the management of prostate cancer is its association with fewer complications while eradicating the cancer. Focal therapy for prostate cancer is a minimally invasive treatment that can be performed under conscious sedation or with spinal anesthesia without the need to admit patients overnight. Patients can return to their normal life immediately after the procedure. Another advantage of focal therapy is that it does not close the door to potential future whole-gland therapies. One of the main disadvantages of focal therapy is the multifocality of prostate cancer. Prostate cancer is multifocal 80% of the time and focal therapy can only address the index lesion, which is typically the focus with the highest Gleason score and with the largest volume. There is growing evidence that the index lesion determines the prognosis of the patient and, therefore, it may be adequate to ablate the index lesion. Nevertheless, the main goal of focal therapy should be to treat patients with intermediate-risk prostate cancer who otherwise would need whole-gland therapy instead of treating patients with low-risk cancer who could benefit from active surveillance.
MR imaging–guided focal treatments
The most commonly used and studied methods of focal therapy for prostate cancer are cryotherapy, high-intensity focused ultrasound (HIFU), and laser ablation. These treatments offer patients alternatives to prostatectomy or radiation therapy and have been found efficacious in treating localized prostate cancer in a comparatively less-invasive manner. Patients undergoing MR imaging–guided tissue-sparing treatments, such as cryotherapy, HIFU, and focal laser ablation, are not entirely spared from the possibility of experiencing adverse side effects, among which are injury to the urethra, nerve damage, and bowel injury. Preventative measures, however, discussed in greater detail later, are taken during the course of all of these treatment procedures and aid in reducing such untoward sequelae.
Cryotherapy
Consisting of a freezing method used to achieve cellular disruption, cryotherapy is an increasingly used treatment method for prostate cancer. Using a transperineal approach, needles are placed in the prostate and the gland is cooled using argon probes. Thermocouples monitor the patient’s body temperature and a transurethral Foley catheter is placed in an effort to prevent urinary complications, including urethral sloughing and incontinence. Saline instilled into the perirectal space has a protective effect on the rectum. Additionally, thermosensors are placed at several anatomic sites, among them the external anal sphincter, the prostate apex, and the neurovascular bundles.
Cryotherapy causes cell apoptosis through the formation of ice crystals at the target site. As a result of the directed cold temperatures, there are cellular dehydration and destruction. Several treatment variables have been found to contribute to a more successful post-therapy outcome: the lowest temperature reached during treatment (a temperature measuring <−40°C is ideal), the rate at which cooling occurs during the freezing step, the length of time during which the freezing occurs, the velocity at which thawing takes place, the number of freeze-thaw cycles used (more extensive tissue damage is typically seen with the incorporation of a double freeze-thaw cycle), and whether there are enlarged vessels present. During the cooling phase, argon gas is used, and helium gas is used in the thawing phase.
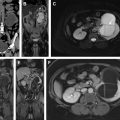
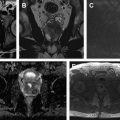
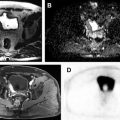
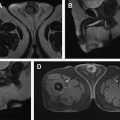
Use of multiparametric MR imaging for guidance when performing cryotherapy has proved more beneficial than using the traditional method of TRUS imaging. In the latter case, real-time evaluation during treatment may be impeded by the reflection of sound waves adjacent to the ultrasound probe, leading to an inaccurate assessment of the zone of treatment. Additionally, the ice ball formed during cryotherapy is better visualized on MR imaging than on sonography. Typical findings on follow-up MR imaging after cryotherapy include the appearance of hypovascularity and focal signal void at the site of treatment. See Fig. 1 for an example of a postprocedural MR imaging appearance of the prostate after cryotherapy of a discrete cancerous lesion.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree