Key points
- •
MR imaging has been reported to be particularly successful in the differentiation of Ta-T1 tumors from T2 and higher staged tumors with 90% sensitivity and 88% specificity in a recent metaanalysis.
- •
MR imaging is still limited in the accuracy for the identification of involved lymph nodes, and the sensitivity and specificity of MR imaging in the identification of involved lymph nodes are 56% and 94 in a recent metaanalysis.
- •
PET-MR imaging could be helpful for staging of bladder cancer; however, there are very limited data in the literature to determine the added value of PET-MR imaging in bladder cancer staging, although it has the potential to improve the accuracy for T and N staging.
Introduction
Multidetector computed tomography (CT) techniques including CT urography have been the most commonly used cross-sectional imaging modality for the evaluation of urinary bladder for the last 2 decades. However, CT scanning is limited in the assessment of bladder owing to low soft tissue contrast resolution and this particularly prevents the detection of small lesions, including but not limited to sessile or flat lesions. The lack of adequate distension and inability to differentiate inflammatory and postbiopsy treatment changes from the tumor and normal bladder wall in addition to low soft tissue contrast resolution lead to a low accuracy of CT scanning in T staging of bladder cancer ranging from 35% to 55% with associated 10% to 39% understaging and 6% to 34% overstaging.
MR imaging has been recently getting increasing attention for the evaluation of bladder cancer not only due to the limitations of CT scans, but also due to its higher soft tissue contrast resolution, and the use of functional MR imaging techniques, including diffusion-weighted imaging (DWI) and dynamic contrast enhanced (DCE) techniques, which have the potential to increase the accuracy of MR imaging compared with previously used conventional sequences. However, adequate bladder distension is necessary to be able to achieve greater accuracy on MR imaging, similar to CT scanning.
Bladder cancer is the sixth most common among all cancers, the fourth most common cancer among men, and the second most common in the genitourinary tract after prostate cancer. Although cystoscopy and transurethral resection of bladder tumor have been reported to be the most accurate ways of detection and characterization of muscle invasiveness of the bladder tumors, these techniques still can understage and overstage the bladder cancer and approximately one-third of invasive cancers have been reported to be understaged. Although the specific role of MR imaging staging has not been defined yet, MR imaging has been reported to provide encouraging results to stage bladder cancer. Additionally, PET-MR hybrid imaging has the potential to contribute to staging of bladder cancer, although there have been no sufficient data in the literature, to date.
Thus, in this review article, the role of MR imaging in the assessment of urinary bladder, particularly bladder cancer with possible potential role of PET-MR imaging in staging, is discussed.
Bladder cancer
Epidemiology
Bladder cancer is the most common malignancy of urinary tract. About 81,190 new cases of bladder cancer (4.7% of all new cancers) and 17,240 deaths from bladder cancer (2.8% of all cancer deaths) are expected in 2018. The 5-year survival rate for bladder cancer is 76.8% between 2008 and 2014.
Urothelial carcinoma, formerly known as transitional carcinoma, is the most common subtype, accounting for 90% of the bladder cancers; the remaining subtypes include squamous cell carcinomas and adenocarcinomas, forming 6% to 8% and 2% of all bladder cancers, respectively, in the Western world. In the developing world, nonurothelial etiologies have a higher incidence compared with the Western world, at least partly owing to schistosomiasis.
Histopathology and TNM Staging
Urothelial carcinoma has been classified into subgroups based on the extent of invasion into the deep layers of bladder wall and surrounding tissues, including non–muscle-invasive urothelial carcinoma and muscle-invasive urothelial carcinoma, because this differentiation has significant therapeutic and prognostic implications.
Bladder cancer is staged according to 2017 American Joint Committee on Cancer TNM staging system https://www.cancer.net/cancer-types/bladder-cancer/stages-and-grades .
Non–muscle-invasive urothelial carcinoma is more common and constitutes 70% of urothelial cancers, whereas muscle-invasive urothelial cancer constitutes 30%. In addition to depth of invasion, urothelial carcinoma was classified into low grade or high grade based on the degree of nuclear anaplasia and architectural abnormalities.
Non–muscle-invasive tumors arise from the urothelium and do not invade the muscularis propria. This subtype includes Ta lesions, which are papillary (exophytic), and low-grade lesions arising from the mucosa; Tis lesions, which are known as carcinoma in situ representing high-grade lesions located in the mucosa; and T1 lesions, which are usually high-grade superficial tumors invading the lamina propria or submucosa of the bladder wall.
Muscle-invasive tumors are almost always high-grade tumors, and include T2 tumors characterized by invasion into the muscularis propria, T3 tumors characterized by invasion into the perivesical fat, and T4 tumors characterized by invasion into the prostate, vagina, uterus, bowel, abdominal/pelvic walls, and other distant organs.
Squamous cell carcinomas and adenocarcinomas are aggressive tumors and usually present with advanced disease.
Initial Approach to the Diagnosis and the Role of Imaging
Gross or microscopic hematuria is the initial typical presentation of bladder cancer, although lower urinary tract symptoms including frequency, urgency, or dysuria could also be the presenting symptoms, albeit less commonly. Evaluation of the bladder and upper urinary tract is essential in the clinical assessment and workup of these symptoms to exclude a urinary tract malignancy.
Cystoscopy is the gold standard for the evaluation of the bladder in these groups of patients to identify and exclude bladder cancer, and is usually combined with urine cytology. Cystoscopy is particularly helpful for the detection of small lesions including flat lesions, which would not be identified with imaging techniques. However, its main advantage is not only the ability to do histopathologic sampling, but also the ability to do transurethral resection of the tumors to determine the depth of invasion, and for therapeutic purposes. Non–muscle-invasive urothelial carcinoma is usually an indolent tumor and can be treated with transurethral resection, intravesical immunotherapy, or intravesical chemotherapy. However, muscle-invasive urothelial carcinoma requires more extensive treatment including cystectomy or multimodality treatment including chemotherapy and radiation therapy followed or preceded by cystectomy.
Cross-sectional imaging with CT urography or MR imaging urography with intravenous contrast is performed to identify concurrent upper tract disease in all patients with bladder cancer. If the bladder cancer is muscle-invasive urothelial cancer or other rare types of bladder cancer are present, cross-sectional imaging is also performed with additional purposes including the assessment of the extension of the pelvic disease, that is, locoregional staging and distant metastases. However, local T staging is very limited with CT scanning and the purpose of CT scanning is usually to evaluate regional lymph nodes for involvement and distant organ metastases. Although promising results have been obtained with MR imaging for local staging of bladder cancer, its specific role in the diagnostic and staging algorithm has not been determined yet.
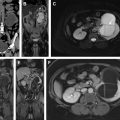
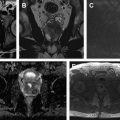
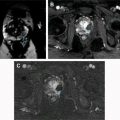
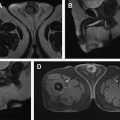
MR imaging has been recently reported to differentiate non–muscle-invasive urothelial carcinoma from the muscle-invasive urothelial carcinoma with 94% accuracy, and this process could be helpful for treatment planning because understaging remains a problem with biopsy and transurethral resection. Therefore, the differentiation of muscle-invasive tumors from non–muscle-invasive tumors may lead to the use of timely and appropriate treatment of muscle-invasive tumors.
MR imaging staging of bladder cancer
MR Imaging Technique
No specific patient preparation is needed. The patient should not void 2 hours before the examination and/or drink 500 mL of water 1 hour before the examination.
Staging MR imaging could be performed at 1.5 T or 3.0 T with phased-array body coils. Although there are still no sufficient data for the comparison of 1.5 T versus 3.0 T for local staging of bladder cancer, it has been reported that 3.0 T may provide higher accuracy in imaging of bladder cancer compared with 1.5 T, which likely results from higher signal-to-noise ratio compared with 1.5 T, particularly for high-resolution T2-weighted imaging and DWI.
MR imaging protocol includes transverse, coronal, and sagittal single shot echo train spin echo T2-weighted sequences; transverse fat-suppressed single shot echo train spin echo T2-weighted sequence; transverse in-phase and out-of-phase dual echo spoiled gradient echo sequence; transverse, coronal, and sagittal high-resolution T2-weighted turbo spin echo (TSE); transverse breathing-independent DWI at b values of 0 and 1000 s/mm 2 , and transverse DCE 3-dimensional gradient echo sequence at arterial phase (at 20–25 seconds after the injection), venous phase (at 60 seconds after the injection), and interstitial phase (at 120 seconds after the injection) followed by coronal and sagittal acquisitions. The details of the MR imaging protocol are given in Table 1 .
| Sequence | Plane | TR | TE | Flip Angle | Thickness/Gap | FOV | Matrix |
|---|---|---|---|---|---|---|---|
| Localizer | 3-plane | ||||||
| SS-ETSE | Coronal | 1500 a | 85 | 170 | 6 mm/20% | 350–400 | 192 × 256 |
| SS-ETSE | Axial | 1500 a | 85 | 170 | 6 mm/20% | 350–400 | 192 × 256 |
| SS-ETSE | Sagittal | 1500 a | 85 | 170 | 6 mm/20% | 350 | 192 × 256 |
| SS-ETSE fat suppressed | Axial | 1500 a | 85 | 170 | 8–10 mm/20% | 350–400 | 192 × 256 |
| T1 SGE in/out of phase | Axial | 170 | 2.2/4.4 | 70 | 7 mm/20% | 350–400 | 192 × 320 |
| T2 3D TSE | Axial | 1200 | 120 | 150 | 1.5 mm | 250 | 256 × 256 |
| T2 TSE | Axial/coronal/sagittal | 5000 | 80 | 90 | 3 mm | 230 | 256 × 256 |
| Diffusion-weighted imaging | Axial | 4500 | 88 | 90 | 3 mm | 270 | 128 × 128 |
| T1 3D GE FS pre | Axial | 3.8 | 1.7 | 10 | 3 mm | 350–400 | 160 × 256 |
| Postgadolinium sequences | |||||||
| T1 3D GE fat suppressed | Axial/coronal/sagittal | 3.8 | 1.7 | 10 | 3 mm | 350–400 | 160 × 256 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree