The newer magnetic resonance (MR) imaging methods can give insights into the initiation, progression, and eventual treatment of osteoarthritis. Sodium imaging is specific for changes in proteoglycan (PG) content without the need for an exogenous contrast agent. T1ρ imaging is sensitive to early PG depletion. Delayed gadolinium-enhanced MR imaging has high resolution and sensitivity. T2 mapping is straightforward and is sensitive to changes in collagen and water content. Ultrashort echo time MR imaging examines the osteochondral junction. Magnetization transfer provides improved contrast between cartilage and fluid. Diffusion-weighted imaging may be a valuable tool in postoperative imaging.
Osteoarthritis (OA) has become the most prevalent chronic disease of the elderly and is an important cause of disability in our society, with increasing incidence not only in the United States but in other parts of the world. It is primarily a disease of the articular cartilage, which may become pathologic by degeneration or acute injury. Because the incidence of the disease is continually increasing, there is a need for accurate noninvasive evaluation before the onset of irreversible changes. There are many diagnostic imaging methods for evaluation of the articular cartilage ( Table 1 ). Conventional radiography has been used to detect secondary gross changes of the joint cartilage, manifested by the narrowing of the joint space distance, and allows visualization of secondary changes, such as osteophyte formation, but this imaging method only allows detection of later stage of the disease when changes are already irreversible. It does not allow direct visualization of the cartilage. Conventional or computed tomography arthrography has also been used to evaluate surface irregularities of the cartilage; however, it is limited in its invasiveness and provides limited evaluation.
| Pros | Cons | |
|---|---|---|
| Sodium imaging | High specificity for PG content. High contrast image without exogenous contrast agent | Requires special coils, high-field scanners, and long imaging times |
| T1ρ imaging | Sensitive to early PG depletion | Requires high RF power. SAR limits |
| dGEMRIC | High resolution and sensitivity | Delay before imaging. Need for contrast agent. Possibility of nephrogenic systemic fibrosis in patients with kidney problems |
| T2 mapping | Sensitive to collagen matrix, water content, and motion | May be less sensitive in detection of early degeneration |
| Ultrashort echo time imaging | Only technique to examine osteochondral junction | Technical challenges. Disadvantage of scan time. Difficulty in slice selection |
| Magnetization transfer | Improved contrast between cartilage and fluid; detection of localized cartilage lesions | Difficult quantification. SAR limits |
| Diffusion-weighted imaging | Postoperative evaluation | Low SNR and spatial resolution |
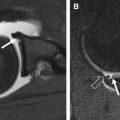
MR imaging has become the best imaging modality for assessment of the articular cartilage because of its ability to manipulate contrast to highlight different tissue types. Conventional MR imaging sequences that are currently used for evaluation of cartilage have the ability to depict mostly morphologic changes, such as fibrillation and partial-thickness or full-thickness defects ; however, they are limited in their capability for comprehensive assessment of cartilage, with limited spatial resolution and limited information about cartilage physiology.
Commonly used conventional MR imaging methods include two-dimensional or multislice T1-weighted, proton density (PD)-weighted, and T2-weighted imaging with or without fat suppression. New developments in imaging hardware and software include improved gradients and radiofrequency coils, fast or turbo spin echo imaging techniques such as water-only excitation. Although spoiled gradient recalled (SPGR) and gradient echo (GRE) techniques have produced excellent images with high resolution (0.3×0.6×1.5 mm) and three-dimensional (3D) SPGR is considered the current standard for morphologic imaging of cartilage, these methods have the disadvantages of lack of reliable contrast between cartilage and fluid and long imaging times.
Therefore, newer techniques have emerged for morphologic imaging of cartilage, some of which include dual-echo steady-state (DESS) imaging, driven equilibrium Fourier transform (DEFT) imaging, balanced steady-state free precession (SSFP) imaging with fat suppression and its variants, such as fluctuating equilibrium MR imaging (FEMR), linear combination (LC) SSFP, incremental decrease in end points through aggressive lipid lowering (IDEAL) SSFP, phase-sensitive SSFP, and vastly interpolated projection reconstruction (VIPR) imaging. These newer methods based on SSFP, as well as advances in parallel technology with improved imaging times in 3D FSE imaging, have improved the contrast, resolution, and acquisition time of morphologic imaging of cartilage. However, these techniques are still limited in their ability to depict physiology and biochemistry of cartilage, although they allow time for the application of other sequences to explore cartilage physiology.
To understand the principles of MR imaging of cartilage physiology, this article reviews some of the basic concepts of cartilage anatomy and physiology.
Functional anatomy and physiology of cartilage
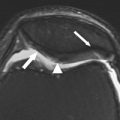
Articular cartilage is hypocellular and composed of about 4% chondrocytes by wet weight. The main component of the tissue is the extracellular matrix, which is 65% to 85% water, which decreases slightly with depth from the articular surface, and solid components, which include type II collagen (15%–20%) and large aggregating molecules of proteoglycans (PGs) (3%–10%), which are called aggrecans. Most of the extracellular water is associated with the aggrecan molecules and freely exchangeable with synovial fluid, whereas a small portion of water is bound in the interfibrillar space of the collagen fibrils ( Fig. 1 A).