Cervical cancer is a significant cause of morbidity and mortality worldwide despite advances in screening and prevention. Although cervical cancer remains clinically staged, the 2009 International Federation of Gynecology and Obstetrics committee has encouraged the use of advanced imaging modalities, including MR imaging, where available, to increase the accuracy of staging, guide treatment, and detect recurrence. Understanding the multiple roles of advanced imaging in the evaluation of cervical cancer will help radiologists provide an accurate and useful report to the referring clinicians.
Key points
- •
Cervical cancer is the second most frequent cause of cancer-related death for women worldwide.
- •
Cervical cancer is clinically staged using the International Federation of Gynecology and Obstetrics (FIGO) classification system, which suffers from poor accuracy in higher-stage disease.
- •
It is now well established that pretreatment imaging with MR imaging and PET/computed tomography (CT) improves patient outcomes.
- •
MR imaging is highly accurate for staging cervical cancer that is stage IB or greater, and FIGO supports the use of MR imaging in conjunction with PET/CT to increase the accuracy of staging, in areas where it is available.
Introduction
Cervical cancer is the third most common cancer in women worldwide and accounts for more than 300,000 deaths annually. Eighty-five percent of cervical carcinomas are the squamous cell subtype, with adenocarcinoma, adenosquamous carcinoma, and undifferentiated carcinoma constituting the remaining 15%. Most cervical cancers are caused by the sexually transmitted human papillomavirus (HPV), with greater than 70% cancers associated with high-risk subtypes HPV-16 and HPV-18. Increased compliance with screening Pap smears for the detection of precancerous lesions, as well as the recent US Food and Drug Administration approval of HPV prevention vaccines, have drastically decreased the rate of cervical cancer in the United States and other developed regions of the world. Despite a marked decrease in the incidence of cervical cancer, those women who develop the disease have nearly a 40% chance of dying from their disease and cervical cancer remains the number 1 cause of cancer-related death in women younger than 35 years of age. MR imaging plays a significant role in guiding the primary treatment in women diagnosed with cervical cancer and has been shown to improve patient outcomes. In turn, radiologists may substantially contribute not only to the initial assessment of cervical cancer but also to treatment response assessment and surveillance. This article discusses the appropriate use of MR imaging in the diagnosis and treatment of cervical cancer.
Introduction
Cervical cancer is the third most common cancer in women worldwide and accounts for more than 300,000 deaths annually. Eighty-five percent of cervical carcinomas are the squamous cell subtype, with adenocarcinoma, adenosquamous carcinoma, and undifferentiated carcinoma constituting the remaining 15%. Most cervical cancers are caused by the sexually transmitted human papillomavirus (HPV), with greater than 70% cancers associated with high-risk subtypes HPV-16 and HPV-18. Increased compliance with screening Pap smears for the detection of precancerous lesions, as well as the recent US Food and Drug Administration approval of HPV prevention vaccines, have drastically decreased the rate of cervical cancer in the United States and other developed regions of the world. Despite a marked decrease in the incidence of cervical cancer, those women who develop the disease have nearly a 40% chance of dying from their disease and cervical cancer remains the number 1 cause of cancer-related death in women younger than 35 years of age. MR imaging plays a significant role in guiding the primary treatment in women diagnosed with cervical cancer and has been shown to improve patient outcomes. In turn, radiologists may substantially contribute not only to the initial assessment of cervical cancer but also to treatment response assessment and surveillance. This article discusses the appropriate use of MR imaging in the diagnosis and treatment of cervical cancer.
MR imaging in the diagnosis and treatment of cervical cancer
Cervical cancer is a clinically staged cancer based on the International Federation of Gynecology and Obstetrics (FIGO) classification system, recently revised in 2009 ( Table 1 ). The initial clinical stage is assigned after gynecologic bimanual and speculum examinations, colposcopy, and cervical biopsy. In regions where MR imaging and PET/computed tomography (CT) are not available, additional invasive techniques, including an examination under anesthesia, cystoscopy, intravenous urography, and sigmoidoscopy or barium enema, can be performed when assessing for advanced disease. Although clinical staging is fairly accurate in early-stage disease, approaching 85% in stage IA to IB1, accuracy in later-stage disease decreases significantly, to less than 35% in stage IIA and 21% in stage IIB when correlated with surgical findings. MR imaging can accurately assess for important prognostic indicators such as tumor size, parametrial invasion, pelvic sidewall invasion, and lymph node metastasis, and has been shown to have accuracy up to 95% for stage IB or greater. As such, FIGO supports the use of MR imaging in conjunction with PET/CT to increase the accuracy of staging, in areas where available.
| Stage | Description | Subtype | Subtype Divisions |
|---|---|---|---|
| I | Confined to the uterus | IA: invasive carcinoma diagnosed only with microscopy with deepest invasion ≤5 mm and largest extension ≤7 mm | IAI: stromal invasion ≤3 mm |
| IA2: stromal invasion >3 mm and ≤5 mm | |||
| IB: clinically visible lesions greater than stage IA | IBI: clinically visible lesion ≤4 cm in greatest dimension | ||
| IB2: clinically visible lesion >4 cm in greatest dimension | |||
| II | Carcinoma invades beyond the uterus, but not to the pelvic wall or lower one-third of the vagina | IIA: without parametrial involvement | IIA1: clinically visible lesion ≤4 cm in greatest dimension |
| IIA2: clinically visible lesion >4 cm in greatest dimension | |||
| IIB: obvious parametrial invasion | |||
| III | Tumor extends to the pelvic wall, involves the lower one-third of the vagina, and/or causes hydronephrosis or a nonfunctioning kidney | IIIA: tumor involves the lower one-third of the vagina | |
| IIIB: extension to the pelvic wall, hydronephrosis, or nonfunctioning kidney | |||
| IV | Tumor extends beyond the true pelvis or has involved the bladder or rectum | IVA: spread of growth to adjacent organs (mucosal involvement of bladder or rectum) | |
| IVB: spread to distant organs (including peritoneal, supraclavicular, mediastinal, or para-aortic lymph nodes) |
Treatment decisions for patients with cervical cancer are determined by the FIGO clinical stage ( Fig. 1 ). Patients with early-stage disease, tumor confined to the uterus, and tumors less than 4 cm (stage I–IB1), are treated with primary surgical resection and lymphadenectomy. Many centers treat women with more advanced-stage disease, defined as stage IB2 or greater, using concurrent chemoradiation as definitive therapy without surgery. This approach is a recent change from previous recommendations to treat only women with stage IIB or higher with chemoradiation. In addition, women with advanced-stage disease (IVB) are offered palliative chemotherapy and symptom control. MR imaging is valuable in determining size of tumor, parametrial invasion, and local metastasis, whereas PET/CT can identify local and distant metastasis and ensure that the radiation treatment volume encompasses clinically involved and high-risk areas. The ultimate goal of advanced imaging is to appropriately stratify patients into treatment groups, to avoid the morbidity and mortality associated with unnecessary surgery, and to ensure all regions of suspected disease are included in the radiation treatment fields.
Magnetic Resonance Protocol
Patient preparation for pelvic MR imaging for cervical cancer includes steps to minimize artifacts and improve visualization of the cervix. It is recommended that patients fast for 4 to 6 hours before the examination to minimize bowel peristalsis. Some institutions choose to administer antiperistaltic medications to further minimize bowel-related motion artifacts. Having the patient void before imaging can help reduce bladder-related artifacts. In addition, vaginal gel can improve visualization of the cervix. Imaging can be performed on either 1.5-T or 3-T magnets with a dedicated pelvic, cardiac, or torso coil. A comprehensive cervical cancer protocol should include the following sequences: Coronal non-fat saturated T2-weighted single shot fast spin echo (FSE) images, axial non-fat saturated T1-weighted FSE images, Axial or axial oblique non-fat saturated T2-weighted FSE images, axial diffusion weighted images, post-contrast axial and sagittal fat saturated T1-weighted spoiled gradient echo images. An example of an MR protocol is provided in Table 2 . Of note, many experts emphasize the importance of true short-axis views of the cervix for small-field-of-view T2-weighted images, whereas others prefer to acquire this series in the true axial plane for the sake of simplicity and reproducibility.
| Sequence | Coronal T2 | Axial T1 | Sagittal T2 | Axial T2 | Axial DWI | Axial T1 | Sagittal T1 | Axial T1 |
|---|---|---|---|---|---|---|---|---|
| Fat Saturated | No | No | Yes | No | No | Yes | Yes | Yes |
| Time After Injection of Contrast (s) | — | — | — | — | — | 40 | 90 | 180 |
| Sequence | Single-shot fast spin echo | Fast spin echo | Fast recovery fast spin echo | Fast recovery fast spin echo | Echo planar | Spoiled gradient echo | Spoiled gradient echo | Spoiled gradient echo |
| Number of Dimensions | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 |
| TE (ms) | 90 | 9 | 90 | 86 | 65 | 1.8 | 1.8 | 1.8 |
| TR (ms) | 2400 | 600 | 3400 | 3400 | 10,000 | 3.8 | 3.8 | 3.8 |
| Echo Train Length | — | 4 | 21 | 21 | — | InvPrep TI = 20 | InvPrep TI = 20 | InvPrep TI = 20 |
| Flip Angle (degrees) | 90 | 90 | 90 | 90 | 90 | 12 | 12 | 12 |
| Number of Excitations | 2 | 4 | 3 | 3 | 8 | 1 | 1 | 1 |
| FOV (cm) | 36 | 26 | 26 | 26 | 32 | 28 | 28 | 28 |
| Slice Thickness/interval (mm) | 6/0 | 5/1.5 | 4/1 | 4/1 | 4/1 | 4.2 | 4.2 | 4.2 |
| Matrix Size | 256 × 224 | 256 × 224 | 320 × 256 | 320 × 256 | 160 × 160 | 256 × 192 | 256 × 192 | 256 × 192 |
| b Value (s/mm 2 ) | — | — | — | — | 0, 500 | — | — | — |
| Approximate Acquisition Time (min:s) | 1:15 | 4:36 | 3:30 | 3:30 | 2:50 | 0:27 | 0:27 | 0:27 |
Interpretation
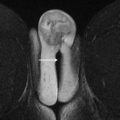
The normal anatomy of the cervix is best visualized on standard fast spin echo T2-weighted images. The normal premenopausal cervix is approximately 3 to 4 cm in length and has a trilaminar appearance: hyperintense endocervical mucosa with a band of low signal intensity representing the cervical stroma deep to the mucosal glands and intermediate-signal-intensity stroma extending to the parametrium ( Fig. 2 A ). The appearance of the cervical stroma changes as the woman ages, becoming more uniformly hypointense in the postmenopausal cervix (see Fig. 2 B).
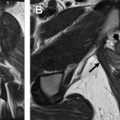
The size, location, and extent of cervical tumors are best evaluated on standard, non-fat saturated T2-weighted and diffusion-weighted images. On T2-weighted images, the tumor shows intermediate to high signal intensity compared with the smooth muscle or myometrium of the uterus ( Fig. 3 A, B ). On diffusion-weighted images, the tumor shows intermediate to high signal on B = 0 images with increase in signal on high B value images and corresponding low signal on apparent diffusion coefficient (ADC) images, reflecting diffusion restriction within the cellular tumor (see Fig. 3 C). Diffusion-weighted images can increase visualization of small tumors that are difficult to delineate on T2-weighted images or in younger patients when the normal cervix is more intermediate in signal intensity, mimicking tumor signal. On dynamic contrast-enhanced images, small tumors show increased early homogeneous hyperenhancement relative to the adjacent normal cervix, whereas larger tumors often show heterogeneous enhancement secondary to necrosis (see Fig. 3 D). Contrast-enhanced images are useful in larger tumors for delineation of tumor extent when evaluating for bladder, rectal, adnexal, or pelvic side wall invasion, or when evaluating for recurrent disease.