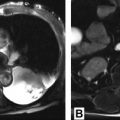
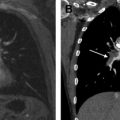
The high soft tissue contrast of MR imaging enables superior tissue characterization of mediastinal masses, adding diagnostic specificity and often changing and benefiting clinical management. MR imaging can better discern cystic from solid content and can detect microscopic fat, hemorrhage, and fibrous content within lesions. In many cases, mediastinal MR imaging may prevent unnecessary diagnostic intervention. In other cases, MR imaging may indicate the optimal site for biopsy or the correct compartment for resection. Awareness of the efficacy of MR imaging with regard to mediastinal mass characterization and judicious MR imaging utilization should further improve patient care.
Key points
- •
The high soft tissue contrast of MR imaging enables superior tissue characterization of mediastinal masses, compared with computed tomography, and often increases diagnostic specificity.
- •
Appropriate use of mediastinal MR imaging may prevent unnecessary diagnostic intervention.
- •
When intervention is needed, mediastinal MR imaging can direct interventionists toward the optimal site for biopsy or the correct compartment for resection.
Introduction
The high soft tissue contrast of MR imaging, relative to other imaging modalities including computed tomography (CT), enables superior tissue characterization of many lesions throughout the body, including those in the mediastinum. The result, in many cases, is added diagnostic specificity or virtual biopsy of the lesion. Much has been written about mediastinal masses and how their differential diagnosis can be narrowed by determination of their mediastinal compartment of origin. The premise of this method is that knowledge of the structures that normally reside in a given mediastinal compartment reduces the number of diagnostic possibilities.
This article describes how adding MR imaging to the diagnostic armamentarium yields further diagnostic precision, with potential to improve clinical management. Therefore, this article is organized not by mediastinal compartment but by MR findings that distinguish one mediastinal mass tissue type from another. The following categories are discussed in the context of their diagnostic significance, as they particularly highlight the value of MR imaging in the mediastinum:
- •
Discernment of cystic from solid lesions
- ○
Corollary: discernment of solid tissue amidst hemorrhage and necrosis; guidance for diagnostic intervention
- ○
- •
Detection of macroscopic and microscopic fat
- •
Detection of lesion T2-hypointensity
- •
Demonstration of a lesion’s dynamic contrast enhancement pattern
- •
Demonstration of matching mediastinal lesions in terms of signal and enhancement in the same patient and its significance
- •
Detection of low apparent diffusion coefficient (ADC) values
- •
Determination of lesion invasiveness
- •
Discernment of mediastinal from paramediastinal lesions
The new International Thymic Malignancy Interest Group (ITMIG) classification of mediastinal compartments, developed by a consensus of its members, is used when describing the compartment of origin of the mass under discussion in the article. Instead of basing lesion location on mediastinal compartments delineated by lines drawn on a lateral chest radiograph, this more modern classification bases lesion location on its relationship to 3 compartments delineated by cross-sectional imaging that extend from the thoracic inlet to the diaphragm: a prevascular (anterior mediastinal) compartment, including all structures anterior to the pericardium and proximal ascending aorta; a visceral (middle mediastinal) compartment, including all major mediastinal visceral structures extending from anterior pericardium posteriorly to a vertical line drawn 1 cm posterior to the anterior margin of the spine (both the trachea and the esophagus are therefore included in this middle mediastinal compartment); and a paravertebral (posterior mediastinal) compartment, including all mediastinal structures posterior to this vertical line. A list of mediastinal masses typically found in each of these compartments is provided in Table 1 .
| Anterior Mediastinum or Prevascular Compartment a | Middle Mediastinum or Visceral Compartment | Posterior Mediastinum or Paravertebral Compartment |
|---|---|---|
| Lymphadenopathy | Lymphadenopathy | Lymphadenopathy |
| Thyroid and parathyroid lesions | Thyroid lesions | — |
| — | Ascending aortic aneurysm Aortic arch aneurysm Dilated main pulmonary artery Aberrant right/left subclavian artery Descending aortic aneurysm | Descending aortic aneurysm |
| Thymic lesions (cyst, hyperplasia, thymic neoplasm, including lymphoma) | — | — |
| — | Paraganglioma and other neurogenic tumors | Neurogenic tumors including neurofibroma, schwannoma, paraganglioma |
| — | — | Lateral meningocele |
| Germ cell tumors | — | — |
| — | Pancreatic pseudocyst | Pancreatic pseudocyst |
| Pleuropericardial or mesothelial b cysts | Mesothelial cysts | Mesothelial cysts |
| — | — | Extramedullary hematopoiesis |
| — | Tracheal lesions | — |
| — | Esophageal lesions | — |
| Morgagni hernia | Hiatal hernia | Bochdalek hernia |
| Bronchogenic cysts (very rarely) | Foregut duplication cysts | Foregut duplication cysts |
| Abscess | Abscess | Abscess |
| Hematoma | Hematoma | Hematoma |
| Fibrosing mediastinitis | Fibrosing mediastinitis | Fibrosing mediastinitis |
| Hemangioma | Hemangioma | Hemangioma |
| Lymphangioma | Lymphangioma | Lymphangioma |
| Sarcoma | Sarcoma | Sarcoma |
a Mediastinal compartments, as prescribed by the new ITMIG classification.
b Mesothelial cysts may be found anywhere in the body where mesothelium exists.
Introduction
The high soft tissue contrast of MR imaging, relative to other imaging modalities including computed tomography (CT), enables superior tissue characterization of many lesions throughout the body, including those in the mediastinum. The result, in many cases, is added diagnostic specificity or virtual biopsy of the lesion. Much has been written about mediastinal masses and how their differential diagnosis can be narrowed by determination of their mediastinal compartment of origin. The premise of this method is that knowledge of the structures that normally reside in a given mediastinal compartment reduces the number of diagnostic possibilities.
This article describes how adding MR imaging to the diagnostic armamentarium yields further diagnostic precision, with potential to improve clinical management. Therefore, this article is organized not by mediastinal compartment but by MR findings that distinguish one mediastinal mass tissue type from another. The following categories are discussed in the context of their diagnostic significance, as they particularly highlight the value of MR imaging in the mediastinum:
- •
Discernment of cystic from solid lesions
- ○
Corollary: discernment of solid tissue amidst hemorrhage and necrosis; guidance for diagnostic intervention
- ○
- •
Detection of macroscopic and microscopic fat
- •
Detection of lesion T2-hypointensity
- •
Demonstration of a lesion’s dynamic contrast enhancement pattern
- •
Demonstration of matching mediastinal lesions in terms of signal and enhancement in the same patient and its significance
- •
Detection of low apparent diffusion coefficient (ADC) values
- •
Determination of lesion invasiveness
- •
Discernment of mediastinal from paramediastinal lesions
The new International Thymic Malignancy Interest Group (ITMIG) classification of mediastinal compartments, developed by a consensus of its members, is used when describing the compartment of origin of the mass under discussion in the article. Instead of basing lesion location on mediastinal compartments delineated by lines drawn on a lateral chest radiograph, this more modern classification bases lesion location on its relationship to 3 compartments delineated by cross-sectional imaging that extend from the thoracic inlet to the diaphragm: a prevascular (anterior mediastinal) compartment, including all structures anterior to the pericardium and proximal ascending aorta; a visceral (middle mediastinal) compartment, including all major mediastinal visceral structures extending from anterior pericardium posteriorly to a vertical line drawn 1 cm posterior to the anterior margin of the spine (both the trachea and the esophagus are therefore included in this middle mediastinal compartment); and a paravertebral (posterior mediastinal) compartment, including all mediastinal structures posterior to this vertical line. A list of mediastinal masses typically found in each of these compartments is provided in Table 1 .
| Anterior Mediastinum or Prevascular Compartment a | Middle Mediastinum or Visceral Compartment | Posterior Mediastinum or Paravertebral Compartment |
|---|---|---|
| Lymphadenopathy | Lymphadenopathy | Lymphadenopathy |
| Thyroid and parathyroid lesions | Thyroid lesions | — |
| — | Ascending aortic aneurysm Aortic arch aneurysm Dilated main pulmonary artery Aberrant right/left subclavian artery Descending aortic aneurysm | Descending aortic aneurysm |
| Thymic lesions (cyst, hyperplasia, thymic neoplasm, including lymphoma) | — | — |
| — | Paraganglioma and other neurogenic tumors | Neurogenic tumors including neurofibroma, schwannoma, paraganglioma |
| — | — | Lateral meningocele |
| Germ cell tumors | — | — |
| — | Pancreatic pseudocyst | Pancreatic pseudocyst |
| Pleuropericardial or mesothelial b cysts | Mesothelial cysts | Mesothelial cysts |
| — | — | Extramedullary hematopoiesis |
| — | Tracheal lesions | — |
| — | Esophageal lesions | — |
| Morgagni hernia | Hiatal hernia | Bochdalek hernia |
| Bronchogenic cysts (very rarely) | Foregut duplication cysts | Foregut duplication cysts |
| Abscess | Abscess | Abscess |
| Hematoma | Hematoma | Hematoma |
| Fibrosing mediastinitis | Fibrosing mediastinitis | Fibrosing mediastinitis |
| Hemangioma | Hemangioma | Hemangioma |
| Lymphangioma | Lymphangioma | Lymphangioma |
| Sarcoma | Sarcoma | Sarcoma |
a Mediastinal compartments, as prescribed by the new ITMIG classification.
b Mesothelial cysts may be found anywhere in the body where mesothelium exists.
Mediastinal magnetic resonance protocol
High quality mediastinal MR protocols involve pulse sequences required to adequately characterize a lesion. These protocols generally include T1-weighted, T2-weighted, and T2-weighted fat-saturated pulse sequences, as well as pre-gadolinium and post-gadolinium three-dimensional (3D) ultrafast gradient echo (GRE) dynamic contrast-enhanced imaging. Ultrafast GRE in-phase and out-of-phase chemical shift MR imaging is recommended for T1-weighted sequences because lesion coverage for both phases can be acquired in a single 20-second breath hold and additional information is obtained regarding the presence or absence of microscopic or intravoxel fat within a lesion at no additional time cost. Supplemental diffusion-weighted and short tau inversion recovery imaging can be performed if the finding of low ADC values is anticipated to refine the differential diagnosis or if the detection of bone marrow edema or involvement is diagnostically or therapeutically critical, respectively.
Breath-hold imaging for all pulse sequences, including the 3-plane localizer, is strongly preferred to respiratory gating because it more reliably freezes respiratory motion and dispenses with associated respiratory motion artifact. Breath-hold imaging is almost universally successful in patients, from adolescent to elderly, provided the technologist rehearses breath-holding with the patient before the patient lies down on the table, primes the patient before each pulse sequence about the nature (length, frequency) of the breath holds, and provides MR-compatible 2-L nasal cannula oxygen when breath-hold difficulty is anticipated. If a patient requires higher amounts of oxygen therapy at home or in the hospital, the volume of oxygen delivery should be suitably adjusted.
When cardiac gating is used, electrocardiogram (ECG) gating is strongly preferred to peripheral gating because ECG gating more reliably freezes cardiac motion and virtually eradicates associated pulsatility artifact.
A suggested mediastinal MR imaging protocol is provided in Table 2 . For noninvasive thymic lesion evaluation, a shortened version of this protocol can be used ( Box 1 ).
| Pulse Sequence | GE | Siemens | Philips | TR (ms) a | TE (ms) b | Flip Angle | NEX | Slice Thickness (mm) |
|---|---|---|---|---|---|---|---|---|
| BH axial and sagittal SSFP balanced gradient echo (GRE) | FIESTA | True FISP | BFFE | 3–270 | 1.2–1.5 (minimum full) | 45–80 | 1 | 7 |
| BH coronal ultrafast spin echo T2 | SSFSE | HASTE | UFSE | 900–1000 (minimum) | 80–100 | NA | ≤1 | 8 |
| BH sagittal UFSE fat-saturated T2 | SSFSE | HASTE | UFSE | 900–1000 (minimum) | 80–100 | NA | ≤1 | 7 |
| BH axial in-phase and out-of-phase (chemical shift) ultrafast GRE T1 (dual echo preferable) | FSPGR | TurboFLASH | TFE | 115–190 | 4.2–4.8/2.1–2.4 | 70–80 | 1 | 7 |
| BH cardiac gated double IR T2 | Double IR FSE T2 | Double IR TSE T2 | Double IR UFSE T2 | 1600–4600 | 110–140 | NA | 1 | 7 |
| BH before and after 3D ultrafast GRE with automated subtraction (post-gadolinium imaging) acquired at 20 s (axial), 1 min (axial), 3 min (sagittal), and 5 min (axial) c upon administration of 20cc of IV gadolinium | LAVA | VIBE | THRIVE | 3–5 | 1.5–2.5 | 10–12 | <1 | 5 |
| Optional coronal/sagittal BH STIR | Fast STIR | Turbo STIR | STIR TSE | 2500 | 50 | NA | 1 | 7 |
| Optional diffusion-weighted echo planar imaging | eDWI | DWI | DWI | 140 | 1–2 (minimum) | NA | 2 | 5–7 |
| Optional BH cardiac gated double inversion recovery T1 | Double IR prep FSE T1 | Double IR prep TSE T1 | Double IR prep UFSE T1 | 850–1100 | 30–40 | NA | 1 | 7 |
| Optional BH cardiac gated sagittal double inversion recovery fat-saturated T2 | Double IR FSE fat-saturated T2 | Double IR TSE fat-saturated T2 | Double IR UFSE fat-saturated T2 | 1600–4600 | 110–115 | NA | ≤1 | 7 |
| Optional respiratory-triggered axial-driven equilibrium without and with fat saturation | FRFSE | RESTORE | DRIVE | 4000–6000 | 90 | NA | 4 | 7 |
a Sample TR: this parameter varies as a function of MR manufacturer, body habitus, desired coverage volume, and many other factors.
b Sample TE: this parameter varies as a function of MR manufacturer, body habitus, desired coverage volume, and many other factors.
c Option to add, for example, postgadolinium axial imaging 2 minutes and 4 minutes after injection. Imaging planes can be changed to optimize lesion characterization.