Magnetic resonance (MR) imaging of the scrotum has been used as a valuable supplemental diagnostic modality in evaluating scrotal pathology, mostly recommended in cases of inconclusive sonographic findings. Because of the advantages of the technique, MR imaging of the scrotum may provide valuable information in the detection and characterization of various scrotal diseases. The technique may accurately differentiate intratesticular from extratesticular mass lesions and provide important information in the preoperative characterization of the histologic nature of scrotal masses. An accurate estimation of the local extent of testicular carcinomas in patients for whom testis-sparing surgery is planned is possible.
Key points
- •
Magnetic resonance (MR) imaging of the scrotum represents a valuable supplemental diagnostic tool in the investigation of scrotal diseases.
- •
The technique is particularly recommended in cases of inconclusive or nondiagnostic sonographic findings.
- •
MR imaging of the scrotum with respect to lesion location, morphology, and tissue characterization provides important information in the presurgical work-up of scrotal masses, improving patient care and decreasing the number of unnecessary surgical explorations.
- •
The technique performs well in the localization of a scrotal mass, in the differentiation of paratesticular masses, in the distinction between benign from malignant intratesticular lesions, and in the evaluation of the local extent of the disease in cases of testicular carcinomas.
Introduction
Imaging has an important role in the investigation of scrotal diseases, although clinical examination represents the primary method for the evaluation of scrotal abnormalities. Sonography currently remains the modality of choice in the initial assessment of scrotal lesions. It is easily performed, widely available, inexpensive, and has been shown to be highly sensitive in the identification of scrotal masses. However, a confident characterization of the nature of intratesticular and paratesticular masses is not always possible, based on sonographic findings only. Magnetic resonance (MR) imaging of the scrotum has been proposed as an alternative imaging technique for the evaluation of scrotal diseases. It is a useful diagnostic tool for the morphologic assessment and tissue characterization in the work-up of scrotal masses and reduces the need for diagnostic surgical explorations of the scrotum. The advantages of MR imaging include simultaneous imaging of both testicles, paratesticular spaces, and spermatic cords; adequate anatomic information; satisfactory tissue contrast; and functional information. MR imaging may be a valuable problem-solving tool in the assessment of scrotal diseases when sonographic findings are equivocal or inconsistent with the clinical findings. Serra and colleagues reported that MR imaging of the scrotum, when performed after inconclusive ultrasound examination, may be diagnostic and cost-effective, although in this study it was required only in 1.4% of cases.
Determining the accurate location of a scrotal mass, whether intratesticular or paratesticular, is important to ensure adequate treatment planning. Most paratesticular masses are benign; therefore, radical orchiectomy may be obviated. MR imaging of the scrotum has been proved highly accurate in the differentiation of extratesticular from intratesticular disease, being superior to sonography especially in cases when the scrotum is markedly enlarged. MR imaging findings with respect to tumor location, morphologic features, and tissue characterization can aid in narrowing the differential diagnosis in cases of paratesticular masses.
Although most intratesticular masses are malignant, a possible diagnosis of various benign intratesticular entities, including dilatation of rete testis, epidermoid cyst, fibrosis, orchitis, infarction, hematoma, and hemorrhagic necrosis, based on MR features may improve patient care and decrease the number of unnecessary radical surgical procedures. In these cases, follow-up, lesion biopsy, tumor enucleation, and testis-sparing surgery (TSS) may be justified. MR imaging of the testicles may provide important information in the preoperative characterization of the histologic nature of various intratesticular mass lesions in terms of morphologic information and by showing the presence of fat, fibrous tissue, fluid, and solid contrast-enhancing tissue within the masses.
According to the current guidelines, radical orchiectomy remains the treatment of choice for testis neoplasms of malignant and unknown origin. Nevertheless, in the last years the management of testicular tumors has started to change in favor of conservative surgery. The widespread use of sonography has led to a marked increase in the number of incidentally detected, small-sized testicular mass lesions, most of which have been proved to be benign. TSS has recently been proposed as an alternative option in selected cases, namely, in small malignant germ cell tumors (GCTs) arising in both or in solitary testes, coupled with local adjuvant radiotherapy and in small Leydig cell tumors, with elective indications (healthy contralateral testes), provided that pathology fails to reveal aggressive features. TSS is also an option in small sonographically detected, nonpalpable tumors provided that histology excludes malignancy (the incidence of benign pathology is reported at approximately 80% in these cases).
Accurate preoperative imaging evaluation of the local stage of disease is mandatory in the care of patients who are candidates for TSS. MR imaging of the scrotum performs well in the evaluation of the local extent of testicular carcinomas. Moreover, MR imaging findings can be closely correlated with the histologic characteristics of testicular neoplasms, providing a preoperative classification of the histologic type of testicular tumors.
Introduction
Imaging has an important role in the investigation of scrotal diseases, although clinical examination represents the primary method for the evaluation of scrotal abnormalities. Sonography currently remains the modality of choice in the initial assessment of scrotal lesions. It is easily performed, widely available, inexpensive, and has been shown to be highly sensitive in the identification of scrotal masses. However, a confident characterization of the nature of intratesticular and paratesticular masses is not always possible, based on sonographic findings only. Magnetic resonance (MR) imaging of the scrotum has been proposed as an alternative imaging technique for the evaluation of scrotal diseases. It is a useful diagnostic tool for the morphologic assessment and tissue characterization in the work-up of scrotal masses and reduces the need for diagnostic surgical explorations of the scrotum. The advantages of MR imaging include simultaneous imaging of both testicles, paratesticular spaces, and spermatic cords; adequate anatomic information; satisfactory tissue contrast; and functional information. MR imaging may be a valuable problem-solving tool in the assessment of scrotal diseases when sonographic findings are equivocal or inconsistent with the clinical findings. Serra and colleagues reported that MR imaging of the scrotum, when performed after inconclusive ultrasound examination, may be diagnostic and cost-effective, although in this study it was required only in 1.4% of cases.
Determining the accurate location of a scrotal mass, whether intratesticular or paratesticular, is important to ensure adequate treatment planning. Most paratesticular masses are benign; therefore, radical orchiectomy may be obviated. MR imaging of the scrotum has been proved highly accurate in the differentiation of extratesticular from intratesticular disease, being superior to sonography especially in cases when the scrotum is markedly enlarged. MR imaging findings with respect to tumor location, morphologic features, and tissue characterization can aid in narrowing the differential diagnosis in cases of paratesticular masses.
Although most intratesticular masses are malignant, a possible diagnosis of various benign intratesticular entities, including dilatation of rete testis, epidermoid cyst, fibrosis, orchitis, infarction, hematoma, and hemorrhagic necrosis, based on MR features may improve patient care and decrease the number of unnecessary radical surgical procedures. In these cases, follow-up, lesion biopsy, tumor enucleation, and testis-sparing surgery (TSS) may be justified. MR imaging of the testicles may provide important information in the preoperative characterization of the histologic nature of various intratesticular mass lesions in terms of morphologic information and by showing the presence of fat, fibrous tissue, fluid, and solid contrast-enhancing tissue within the masses.
According to the current guidelines, radical orchiectomy remains the treatment of choice for testis neoplasms of malignant and unknown origin. Nevertheless, in the last years the management of testicular tumors has started to change in favor of conservative surgery. The widespread use of sonography has led to a marked increase in the number of incidentally detected, small-sized testicular mass lesions, most of which have been proved to be benign. TSS has recently been proposed as an alternative option in selected cases, namely, in small malignant germ cell tumors (GCTs) arising in both or in solitary testes, coupled with local adjuvant radiotherapy and in small Leydig cell tumors, with elective indications (healthy contralateral testes), provided that pathology fails to reveal aggressive features. TSS is also an option in small sonographically detected, nonpalpable tumors provided that histology excludes malignancy (the incidence of benign pathology is reported at approximately 80% in these cases).
Accurate preoperative imaging evaluation of the local stage of disease is mandatory in the care of patients who are candidates for TSS. MR imaging of the scrotum performs well in the evaluation of the local extent of testicular carcinomas. Moreover, MR imaging findings can be closely correlated with the histologic characteristics of testicular neoplasms, providing a preoperative classification of the histologic type of testicular tumors.
MR imaging protocol
MR imaging of the scrotum is performed with the use of a pelvic phased array coil or a surface coil. Patients are examined in the supine position, with the testes placed at a similar distance from the coil, usually by means of a towel placed beneath the testis and the penis draped on the anterior abdominal wall.
The MR imaging protocol should include
- 1.
Thin-section spin-echo T1-weighted images in the transverse plane should be included. These images provide information about scrotal anatomy and demonstrate hyperintense lesions.
- 2.
Axial fat-suppressed T1-weighted sequences are repeated when a lesion with high T1 signal intensity is detected.
- 3.
Thin-section fast spin-echo T2-weighted images in 2 or 3 planes should be included, including the transverse plane and the coronal and/or the sagittal plane. These images are best for lesion detection, localization, and characterization. On the coronal plane, a comparative evaluation of both testes, the paratesticular spaces, and the spermatic cords is possible.
Dynamic contrast-enhanced (DCE) subtracted MR imaging, diffusion-weighted imaging (DWI), and MR spectroscopy (MRS) have recently added important diagnostic information in the investigation of scrotal diseases. DCE MR imaging provides information regarding the characteristics of microvasculature of testicular carcinomas and assesses tumor angiogenesis. In malignancies, DCE MR imaging typically shows rapid and intense enhancement, followed by a relatively rapid washout of the contrast medium; this was also proved for testicular carcinomas. DCE MR imaging has been proved useful in the characterization of scrotal lesions and in the distinction between testicular torsion and trauma from other causes of acute pain. DCE MR imaging in combination with T2- and T2*-weighted images is useful in the diagnosis of testicular torsion and in the detection of testicular necrosis. A sensitivity of 100% was referred for DCE-MR imaging in diagnosing complete torsion by showing a decrease or lack of testicular perfusion. The same group of researchers described the differences of testicular enhancement patterns in 42 patients with a variety of scrotal diseases. They concluded that the relative percentages of peak height and mean slope based on time-signal intensity (TSI) curves may be used to differentiate intratesticular from extratesticular diseases. DCE subtracted MR imaging has also been used for the differential diagnosis between benign and malignant intratesticular lesions. The progression of enhancement was classified according to the shape of the TSI curves into 3 types: type I curve, with a linear increase of contrast enhancement over the entire dynamic period, indicating a benign diagnosis; type II curve, with an initial upstroke, after which the signal intensity either plateaus or gradually increases in the late contrast-enhanced period, also suggestive of benignity; and type III curve, with an initial upstroke, followed by gradual washout of the contrast medium, indicating a diagnosis of malignancy ( Fig. 1 ). The relative percentages of peak height, maximum time to peak, and mean slope were calculated to assess possible independent predictors of malignancy. A significant association between the type of the TSI curve and the final diagnosis was demonstrated, and the relative percentages of maximum time to peak proved to be the most important discriminating factor in characterizing intratesticular masses.
DWI is an evolving technique that can be used to improve tissue characterization when interpreted in combination with the findings of conventional MR sequences. Lesion detection and characterization mainly depends on the extent of tissue cellularity, and increased cellularity is associated with restricted diffusion and reduced apparent diffusion coefficient (ADC) values. A few series reported the usefulness of a high b value DWI in the detection and localization of nonpalpable undescended testes in children, when combined with conventional MR imaging data. The researchers concluded that hypointensity of testicular parenchyma in these patients detected on all sequences, including DWI, may be related to nonviable testis, therefore, preventing unnecessary surgical procedures. Other studies concluded that ADC measurements may be used for the early diagnosis of testicular torsion, without the need of intravenous contrast media, reporting significantly lower mean ADC of the twisted testes than that of the normal contralateral testes because of the presence of ischemia and/or hemorrhagic necrosis. DWI and ADC measurements have been reported to be useful in differentiating between normal, benign, and malignant scrotal contents when interpreted in combination with the conventional MR imaging. By combining DWI ( b = 900 s/mm 2 ) with conventional MR imaging, an accuracy of 100% has been reported in the characterization of scrotal lesions.
The MR imaging protocol for the evaluation of scrotal masses is described in Table 1 .
| Sequences | Parameters |
|---|---|
| Transverse spin-echo T1-weighted In cases of hyperintense lesions on T1-weighted images, repeated with fat saturation | TR/TE (ms): 500–650/13–15 Slice thickness (mm): 3–4 Gap (mm): 0.5 Matrix (mm): 180 × 256 FOV (mm): 240 × 270 |
| Transverse, coronal and sagittal fast-spin echo T2-weighted | TR/TE (ms): 3900/120 Slice thickness (mm): 3–4 Gap (mm): 0.5 Matrix (mm): 180 × 256 FOV (mm): 240 × 270 |
| Transverse DW single-shot, multi-slice spin-echo planar diffusion pulse | TR/TE (ms): 4000/115 Slice thickness (mm): 3–4 Gap (mm): 0.5 Matrix (mm): 180 × 256 FOV (mm): 240 × 270 b value (s/mm 2 ): 0, 900 |
| Coronal dynamic 3D fast-field subtracted postcontrast | TR/TE (ms): 9/4.1 Slice thickness (mm): 4 Gap (mm): 0 Matrix (mm): 256 × 256 FOV (mm): 219 × 219 Flip angle: 35° Dynamic scans: 7 (every 60 s) Contrast material IV (mL/kg): 0.2 |
Proton MRS ( 1 H-MRS) provides chemical data on tissue components and has been recently used for the study of human testes. Firat and colleagues showed differences in 1 H-MRS characteristics between prepubertal and postpubertal male volunteers. An increase of choline peak and a significant decrease of the lipid peak have been reported after puberty, both related to the initiation of spermatogenesis. Aaronson and colleagues showed a significantly high phosphocholine concentration in testes with spermatogenesis, concluding that 1 H-MRS may provide a noninvasive imaging tool in the investigation of male infertility.
Normal anatomy
Normal testes appear homogeneous, with signal intensity similar to that of the surrounding muscle on T1-weighted images ( Fig. 2 ). The internal structure of testicular parenchyma is better appreciated on T2-weighted images. Normal testes appear hyperintense on T2-weighted sequences, although the signal intensity is less than that of fluid (see Fig. 2 ; Fig. 3 ). Thin radiating hypointense septa are often seen through parenchyma toward the mediastinum testis, which is depicted as a low-signal-intensity band in the posterior aspect of the testis. The tunica albuginea appears as a thin hypointense halo surrounding the testis. Normal testis enhances moderately and homogeneously after gadolinium administration (see Fig. 2 E), with a gradual increase of signal intensity throughout the examination (type I curve). This behavior is probably related to an intact blood-testis barrier. Testicular parenchyma has been reported hyperintense and slightly hypointense on high b value DWI and ADC maps, respectively, because of the complexity in histology of normal testicular parenchyma (see Fig. 2 F, G). Densely packed seminiferous tubules, lined by a compact fibroelastic connective tissue sheath and interstitial stroma, containing fibroblasts, blood vessels, lymphatics, and the Leydig cells within normal testis, are responsible for the restricted diffusion. ADC values of the normal testis have been previously published (1.11 ± 0.18 × 10 −3 mm 2 /s).
The normal epididymis appears as isointense on T1- and hypointense on T2-weighted sequences, respectively, and is better appreciated on sagittal T2-weighted images (see Fig. 3 ). It appears with low signal intensity on DWI, and the mean ADC is 1.39 ± 0.14 × 10 −3 mm 2 /s (see Fig. 3 C). A small hydrocele is often seen in the scrotum and is considered a physiologic finding. The spermatic cords are better evaluated on coronal T2-weighted sequences, as hyperintense structures, because of the presence of fat, with hypointense vessels coursing through them (see Fig. 2 C, D).
Pathology
Intratesticular Masses
Testicular tumors
Testicular carcinoma, although representing 1.0% to 1.5% of all malignancies in men, is the most common neoplasm in boys and young adults aged 15 to 34 years old. The estimated number of new cases of testicular cancer in the United States during 2013 is 7920, and deaths related to testicular cancer are estimated to occur in 370 patients. Testicular cancer more often presents as a painless scrotal mass.
Testicular tumors may be subdivided into GCTs and non-GCTs ( Box 1 ). Most (95%) testicular carcinomas are GCTs, arising from the germinal epithelium of the seminiferous tubules. The GCTs are fairly evenly split between seminomas and nonseminomatous GCTs (NSGCTs). Less than 50% of malignant GCTs have a single cell type, of which roughly 50% are seminomas, seen more often during the fourth decade of life. The remaining testicular tumors have more than 1 cell type. NSGCTs include a large group of histologically diverse neoplasms, with 4 basic types, including embryonal carcinoma, teratoma, choriocarcinoma, and yolk sac tumor. NSGCTs typically occur earlier in life, usually during the third decade.
GCTs
Intratubular germ cell neoplasia, unclassified
Malignant pure GCT (showing a single cell type)
Seminoma
Embryonal carcinoma
Teratoma
Choriocarcinoma
Yolk sac tumor
Malignant mixed GCT (showing more than one histologic type)
Embryonal carcinoma and teratoma with or without seminoma
Embryonal carcinoma and yolk sac tumor with or without seminoma
Embryonal carcinoma and seminoma
Yolk sac tumor and teratoma with or without seminoma
Choriocarcinoma and any other element
Polyembryoma
Sex cord and stromal tumors
Leydig cell tumor
Sertoli cell tumor
Granulosa cell tumor
Fibroma-thecoma
Tumors with both sex cord and stromal cells and germ cells
Gonadoblastoma
Lymphoid and hematopoietic tumors
Lymphoma
Leukemia
Metastases
The local extent (T) of testicular tumors is classified according to the recommendations of the European GCC Consensus Group (EGCCCG) and is presented in Table 2 . More than 70% of GCTs are diagnosed at an early stage.
| Stage of Primary Tumor | Extent of Primary Tumor Assessed After Radical Orchiectomy |
|---|---|
| pTx | Primary tumor cannot be assessed |
| pT0 | No evidence of primary tumor in the testis |
| pTis | Intratubular germ cell neoplasia or carcinoma in situ |
| pT1 | Tumor confined to the testis, without vascular or lymphatic invasion, with or without involvement of the epididymis or rete testis: may invade the tunica albuginea but not the tunica vaginalis |
| pT2 | Tumor confined to the testis, with or without involvement of the epididymis or rete testis, with vascular or lymphatic invasion or extension through the tunica albuginea with invasion of the tunica vaginalis |
| pT3 | Tumor invades the spermatic cord with or without vascular or lymphatic invasion |
| pT4 | Tumor invades the scrotal wall with or without vascular or lymphatic invasion |
Most non–germ cell neoplasms of the testis are derived from the cells forming the sex cords (Sertoli cells) and the interstitial stroma (Leydig cells). These neoplasms compose approximately 4% of all testicular malignancies in adults, with a higher incidence in children (10%–30%). Although they are usually benign (90%), the preoperative diagnosis of their nature is usually difficult.
Testicular lymphoma constitutes 1% to 9% of all testicular carcinomas but represents the most common testicular tumor in men older than 60 years. Secondary involvement of the testis in patients with an established lymphoma is much more common than primary testicular lymphoma. It is the most common bilateral testicular neoplasm, often locally invasive, typically infiltrating the epididymis, the spermatic cord, and the scrotal skin. Leukemia may appear as an infiltrative epididymal-testicular mass, more often in patients with a known history of treated leukemia.
MR imaging findings
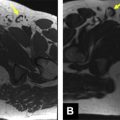
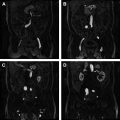
Conventional MR imaging criteria used to characterize testicular malignancies include the presence of a predominantly hypointense intratesticular mass lesion on T2-weighted images when compared with the normal testis ( Fig. 4 ) or a heterogeneous mass with variable signal intensity on T2-weighted images ( Fig. 5 ), inhomogeneously enhancing after gadolinium administration (see Figs. 4 and 5 ). All testicular neoplasms usually have similar signal intensity with the normal contralateral testis on T1-weighted sequences (see Fig. 4 C). The coexistence of areas of hemorrhage and/or necrosis within the tumor (see Fig. 5 ; Fig. 6 ) as well as the extension of the neoplasm to the testicular tunicae ( Fig. 7 ), the paratesticular space (see Fig. 4 ), and/or the spermatic cord ( Fig. 8 ) are considered as secondary findings used to confirm the diagnosis of malignancy.