Pelvic floor dysfunction is a term used to describe a broad set of conditions including pelvic organ prolapse, urinary or fecal incontinence, defecatory dysfunction, and chronic pelvic pain that frequently affects multiple compartments of the pelvic floor. Imaging is important, because physical examination may not be adequate as the only means of evaluating pelvic floor disorders. This article reviews pertinent pelvic floor anatomy as well as the technique for performing, interpreting, and reporting abnormalities seen on MR defecography examinations in the anterior, middle, and posterior compartments.
Key points
- •
Pelvic floor dysfunction typically affects multiple compartments of the pelvic floor.
- •
Knowledge of pelvic floor anatomy is important, and MR imaging allows for direct visualization of anatomic defects.
- •
Instillation of rectal contrast and defecation of the rectal gel during the examination are imperative for adequate MR defecography technique.
- •
Multiple attempts at defecation and/or Valsalva should be performed in order to elicit maximal prolapse.
- •
Pelvic floor MR imaging should include evaluation of anatomic defects and postsurgical changes including those pertaining to previously placed synthetic materials.
Introduction
Pelvic floor disorders affect approximately 25% of women over the age of 20 years and up to 50% of women beyond 50 years old in the United States, with negative impact on quality of life in a significant number of those affected. Patients with pelvic floor dysfunction may present with pelvic pressure from pelvic organ prolapse, urinary or fecal incontinence, defecatory dysfunction, and/or chronic pelvic pain. Risk factors include female gender, advanced age, parity, childbirth, hysterectomy, obesity, connective tissue disorders, smoking, trauma to the pelvic floor, and other conditions that may result in chronic increase in intraabdominal pressure. More than 500,000 surgical procedures are performed in the United States annually for pelvic organ prolapse and urinary incontinence, and the lifetime risk of undergoing a single surgical procedure for either one of these conditions by the age of 80 years is 11%. The direct cost of urinary incontinence in the United States for women in 1995 was estimated at $12 billion. Importantly, the reoperation rate for recurrent prolapse is nearly 30% ; repeat intervention is often performed for occult components of disease that may have not been apparent on initial physical examination.
Physical examination may not be adequate as the sole means to evaluate pelvic floor dysfunction. Imaging can help identify or better grade pelvic floor dysfunction given the complex and multifactorial nature of the condition, which often involves multiple compartments of the pelvic floor. Fluoroscopic defecography has traditionally been used for imaging of pelvic floor dysfunction in the physiologic upright sitting position ; however, it involves exposure to ionizing radiation and does not allow for direct visualization of the pelvic floor anatomy. Depending on the specific technique used, it may not evaluate all 3 pelvic floor components or may require ingestion of oral contrast or instillation of contrast in the bladder and vagina.
Dynamic MR imaging of the pelvis for evaluation of pelvic floor descent was first described by Yang and colleagues in 1991 and has since developed into an important tool for both anatomic and functional evaluation of the pelvic floor. Multiplanar capabilities and high inherent contrast resolution of MR imaging allow for direct visualization of pelvic organs as well as the muscular anatomy of the pelvic floor. Furthermore, all 3 compartments of the pelvic floor can be evaluated in unison without additional patient preparation. High cost, supine positioning during imaging, and substantial variability of pelvic MR imaging measurements have been deterrents to universal adoption of dynamic MR imaging of the pelvic floor. Although MR defecography may show a wide range of findings even in asymptomatic patients, the degree of prolapse on MR imaging has been shown to be higher in symptomatic patients, and MR imaging has been shown to alter surgical management in 67% of patients. Recently published American College of Radiology imaging Appropriateness Criteria consider MR defecography with rectal contrast equivalent to fluoroscopic cystocolpoproctography for evaluation of suspected pelvic floor prolapse and urinary dysfunction. The criteria favor MR imaging for defecatory dysfunction and suspected recurrent prolapse, and for pelvic floor dysfunction following pelvic floor repair. This article reviews pertinent pelvic floor anatomy, appropriate MR imaging techniques for evaluation of the pelvic floor, and findings encountered during interpretation of anatomic and functional pelvic floor MR imaging.
Introduction
Pelvic floor disorders affect approximately 25% of women over the age of 20 years and up to 50% of women beyond 50 years old in the United States, with negative impact on quality of life in a significant number of those affected. Patients with pelvic floor dysfunction may present with pelvic pressure from pelvic organ prolapse, urinary or fecal incontinence, defecatory dysfunction, and/or chronic pelvic pain. Risk factors include female gender, advanced age, parity, childbirth, hysterectomy, obesity, connective tissue disorders, smoking, trauma to the pelvic floor, and other conditions that may result in chronic increase in intraabdominal pressure. More than 500,000 surgical procedures are performed in the United States annually for pelvic organ prolapse and urinary incontinence, and the lifetime risk of undergoing a single surgical procedure for either one of these conditions by the age of 80 years is 11%. The direct cost of urinary incontinence in the United States for women in 1995 was estimated at $12 billion. Importantly, the reoperation rate for recurrent prolapse is nearly 30% ; repeat intervention is often performed for occult components of disease that may have not been apparent on initial physical examination.
Physical examination may not be adequate as the sole means to evaluate pelvic floor dysfunction. Imaging can help identify or better grade pelvic floor dysfunction given the complex and multifactorial nature of the condition, which often involves multiple compartments of the pelvic floor. Fluoroscopic defecography has traditionally been used for imaging of pelvic floor dysfunction in the physiologic upright sitting position ; however, it involves exposure to ionizing radiation and does not allow for direct visualization of the pelvic floor anatomy. Depending on the specific technique used, it may not evaluate all 3 pelvic floor components or may require ingestion of oral contrast or instillation of contrast in the bladder and vagina.
Dynamic MR imaging of the pelvis for evaluation of pelvic floor descent was first described by Yang and colleagues in 1991 and has since developed into an important tool for both anatomic and functional evaluation of the pelvic floor. Multiplanar capabilities and high inherent contrast resolution of MR imaging allow for direct visualization of pelvic organs as well as the muscular anatomy of the pelvic floor. Furthermore, all 3 compartments of the pelvic floor can be evaluated in unison without additional patient preparation. High cost, supine positioning during imaging, and substantial variability of pelvic MR imaging measurements have been deterrents to universal adoption of dynamic MR imaging of the pelvic floor. Although MR defecography may show a wide range of findings even in asymptomatic patients, the degree of prolapse on MR imaging has been shown to be higher in symptomatic patients, and MR imaging has been shown to alter surgical management in 67% of patients. Recently published American College of Radiology imaging Appropriateness Criteria consider MR defecography with rectal contrast equivalent to fluoroscopic cystocolpoproctography for evaluation of suspected pelvic floor prolapse and urinary dysfunction. The criteria favor MR imaging for defecatory dysfunction and suspected recurrent prolapse, and for pelvic floor dysfunction following pelvic floor repair. This article reviews pertinent pelvic floor anatomy, appropriate MR imaging techniques for evaluation of the pelvic floor, and findings encountered during interpretation of anatomic and functional pelvic floor MR imaging.
Pelvic floor anatomy
A basic understanding of pelvic floor anatomy is essential for adequate anatomic and functional evaluation of the pelvic floor on MR imaging. For purposes of clinical evaluation, the female pelvis is classically divided into 3 compartments: the anterior compartment containing the bladder and urethra; the middle compartment containing the uterus, cervix, and vagina; and the posterior compartment containing the rectum and anal canal. These compartments are closely interrelated, and patients often present with multicompartment dysfunction. The compartments of the pelvic floor are supported by a complex network of fascia, ligaments, and pelvic floor muscles that form 3 layers of support: the endopelvic fascia (superior), the pelvic diaphragm (middle), and the perineal membrane or urogenital diaphragm (inferior). The fascia and ligaments provide passive support, whereas the musculature of the pelvic diaphragm provides the underlying tone and can be recruited for active support.
Endopelvic Fascia
The endopelvic fascia is a sheet of connective tissue that extends across the pelvic floor from the bony pelvis on one side to the other and forms the superior-most layer of support of the pelvic floor. It covers the levator ani muscles and pelvic viscera. Various components of the endopelvic fascia are named according to their location. The pubocervical fascia between the bladder and vagina or cervix, the parametrium extending from the cervix to the lateral sidewalls, the paracolpium extending from the vagina to the pelvic sidewalls, and the rectovaginal fascia between the vagina and rectum may not be visible on standard MR imaging. Other components such as the urethral ligaments and perineal body are identifiable on MR imaging. The cardinal and uterosacral ligaments arise from condensations of the endopelvic fascia superiorly. Laterally, the endopelvic fascia coalesces to form the arcus tendineus along the bony pelvis, which serves as an attachment site for the muscles that form the pelvic diaphragm. The endopelvic fascia provides 3 levels of support in relation to vagina: level I (vaginal apex), level II (mid vagina), level III (distal vagina), and defects in each level may present with unique physical signs and symptoms. Deficiencies within different portions of the fascia may determine the degree of prolapse in each compartment. For example, defects in the uterosacral ligaments or the paracolpium/parametrium may result in cervical or vaginal prolapse; tears in the pubocervical fascia or urethral ligaments may result in cystocele and urethral hypermobility; and defects in the perineal body or rectovaginal fascia may present with anterior rectocele or enterocele.
Pelvic Diaphragm
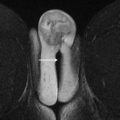
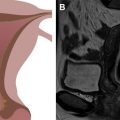
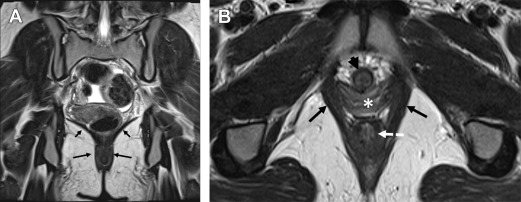
The pelvic diaphragm provides the middle layer of support of the pelvic floor and consists of the ischiococcygeus muscles and the levator ani muscles. The levator ani is a vaselike or hourglass-shaped group of striated muscles composed of the iliococccygeus, pubococcygeus, and puborectalis muscles ( Fig. 1 ). These muscles are well seen on MR imaging and normally maintain a convex appearance superiorly. The relatively thin iliococccygeus muscle attaches anteriorly to the pubic bone, laterally to the arcus tendineus, and inferiorly to the external anal sphincter. The thicker pubococcygeus muscle is more medially located relative to the iliococccygeus; it arises from the superior pubic rami and wraps around the bladder, urethra, vagina, and rectum. Posteriorly, the iliococccygeus and pubococcygeus form a thick condensation of tissue called the levator plate ( Fig. 2 ), which inserts upon the sacrum and coccyx. The iliococccygeus and pubococcygeus muscles are difficult to differentiate on MR imaging owing to the overlap in fibers and morphology. The most caudal component of the levator ani muscle group is the puborectalis, which attaches anteriorly to the pubic symphysis and forms a U-shaped sling around the anorectum (see Fig. 1 ). The level of the puborectalis impression upon the posterior rectum demarcates the anorectal junction, and the margins of the puborectalis form the urogenital or pelvic floor hiatus. Superiorly, the puborectalis muscle fibers overlap with the pubococcygeus. The ischiococcygeus or the coccygeus muscle is a relatively minor part of the pelvic diaphragm, extending from the coccyx in the midline to the ischial spine bilaterally. The pelvic diaphragm provides continuous tone to the pelvic floor, but can be contracted or relaxed actively. Atrophy or defects of the pelvic diaphragm, particularly the puborectalis, are well depicted on axial MR imaging ( Fig. 3 ).
Perineal Membrane/Urogenital Diaphragm
The perineal membrane (also referred to as the urogenital diaphragm) forms the caudal-most layer of the pelvic floor and comprises primarily the deep transverse perineal muscle and connective tissue that extend from ischial rami laterally to the perineal body in the midline ( Fig. 4 ). The perineal membrane attaches anteriorly to the pubic symphysis, giving the perineal membrane a triangular shape.
MR imaging technique
Dynamic MR imaging of the pelvic floor can be performed in an upright low field strength open magnet with the patient sitting on a modified MR imaging–safe commode, or with the patient supine on a conventional high-field-strength magnet (1.5 T or 3 T). The sitting position is more physiologic; however, upright magnets are not readily available at most centers, making upright MR defecography difficult to perform. Alternatively, supine MR defecography can be performed with relative ease on most high-field-strength magnets. MR imaging with defecation in the sitting position may be preferred for evaluation of defecatory dysfunction in the posterior compartment ; however, Gufler and colleagues showed no significant difference in depiction of prolapse in the anterior and middle compartment between supine MR imaging and upright colpocystoproctography. In contrast, Kelvin and colleagues showed an underestimation of the extent of cystoceles and enteroceles on supine MR defecography relative to fluoroscopic cystocolpoproctography; they did, however, note that MR imaging had the advantage of directly visualizing all pelvic organs and musculature in a dynamic fashion. Multiple other studies have also compared upright fluoroscopic defecography or upright MR defecography with supine dynamic pelvic floor MR imaging with variable results. Most of these studies have not used defecation for the supine MR imaging protocol, thus confounding the true effect of positioning on degree of prolapse when comparing the examinations. A recent study comparing MR defecography in both upright and supine positions in the same set of patients showed significantly lower position of the bladder and vagina at defecation during the upright MR imaging, but not of the anorectal junction ; however, imaging in the upright position immediately followed imaging in the supine position in that study, raising the possibility that pelvic floor fatigue could have played a role in eliciting a higher degree of prolapse on the latter examination. Kumar and colleagues actually demonstrated a higher degree of prolapse in the anterior compartment when comparing supine MR imaging with defecation to another upright examination: standing voiding cystourethrogram (VCUG).
Regardless of supine or upright positioning, defecation has been shown to be imperative for dynamic assessment of the pelvic floor. Functional imaging with defecation necessitates instillation of contrast material in the rectum. Although the exact composition and volume of rectal contrast media vary widely in the literature, ultrasound gel is the most commonly used agent due to sterility, relatively low cost, availability, and ease of instillation. The authors use 120 mL of gel in order to avoid overdistention of the rectum while allowing adequate volume for defecation. In their experience, smaller volumes of 60 mL are difficult for the patients to evacuate.
In addition to providing functional evaluation, MR imaging allows for direct visualization of multiple pelvic structures without having to opacify small bowel, bladder, or vagina with contrast. Furthermore, detailed evaluation of the pelvic floor levator muscles is possible on MR imaging. Supine MR defecography, usually performed on a higher-field-strength magnet (1.5 T or 3 T), allows for higher resolution evaluation of the anatomy in comparison to lower-field-strength open magnets, which typically suffer from poor signal-to-noise as well as lower spatial and contrast resolution. The higher resolution provided by supine high-field-strength MR imaging is also beneficial when evaluating previously placed urethral slings or pelvic mesh.
Because of the unusual nature of MR defecography, referring physicians explain the examination to the patients during the clinic visit, and the authors provide patients with educational material about the examination before their arrival to the imaging center. The MR technologists brief the patients and attempt to relieve any anxiety about the examination upon arrival on the day of examination. They specifically coach the patients on how to perform the Kegel (squeeze), strain, and defecation maneuvers before taking the patient to the magnet room. Patients are also instructed to urinate upon arrival and then drink 16 ounces of water in an effort to achieve standardized mild bladder distention. The goal is to partially fill the bladder while avoiding overdistention because this may obscure prolapse in other compartments. The MR imaging table is covered with disposable absorbent pads. Patients are positioned on the MR imaging table with their pelvis centered in an inflatable plastic enema ring. Ultrasound gel is instilled using a catheter tip syringe with patients in the lateral decubitus position, and the patients are then placed again in the supine position for imaging, with knees slightly flexed on a pillow or wedge for support. A multichannel phase-array surface coil is positioned over the patient’s pelvis for MR image acquisition.
T2-weighted (T2w) turbo spin echo (TSE) images in sagittal, axial, and coronal planes and axial T1-weighted gradient echo (GRE) images can be acquired at rest to allow for anatomic evaluation. These should be followed by cine-type true fast imaging with steady state precession (TrueFISP) or single shot fast spin echo (SSFSE) in a single midsagittal plane for functional imaging performed during Kegel (squeeze), strain, and defecation. Although both sequences may perform acceptably, TrueFISP images have been shown to demonstrate higher degrees of prolapse in all 3 compartments than SSFSE images. The authors advocate at least 3 attempts at defecation in order to elicit maximum degree of prolapse. The sagittal cine-type TrueFISP or SSFSE images should be repeated after defecation with a postdefecation strain maneuver, because this may sometimes show a higher degree of prolapse particularly when the patients are unable to completely empty the rectum during the defecation phases. If patients are not able to defecate after 3 attempts, they are instructed to defecate in the restroom and then immediately return to MR imaging for the postdefecation strain images. Optional sequences include cine-type TrueFISP or SSFSE images in coronal oblique or axial oblique planes, along the axis of the anal canal or the pubococcygeal line (PCL), respectively. These are helpful in visualizing para-midline defects that are occult in the single midsagittal plane. Although either 1.5-T or 3-T magnets may be used for MR defecography, the authors find certain sequences such as cine TrueFISP to be more robust with fewer artifacts at 1.5 T. A sample MR defecography protocol is detailed in Table 1 .
| Sequence | Imaging Plane | Maneuver | FOV (cm) | Slice Thickness (mm) | TR (ms) | TE (ms) | Flip Angle (°) | Matrix |
|---|---|---|---|---|---|---|---|---|
| T2 TSE | Axial | Rest | 26 | 5 | 3920 | 91 | 150 | 320 × 256 |
| T2 TSE | Sagittal | Rest | 26 | 5 | 4070 | 91 | 150 | 320 × 256 |
| T2 TSE | Coronal | Rest | 26 | 5 | 5120 | 91 | 150 | 320 × 256 |
| T1 GRE OP/IP | Axial | Rest | 26 | 5 | 140 | 2.3/4.6 | 55 | 256 × 208 |
| Cine TrueFISP | Sagittal | Kegel | 34 | 8 | 734.4 | 1.8 | 80 | 256 × 256 |
| Cine TrueFISP ×3 | Sagittal | Defecation | 34 | 8 | 734.4 | 1.8 | 80 | 256 × 256 |
| Cine TrueFISP | Axial Oblique | Defecation | 33 | 8 | 742.6 | 1.8 | 80 | 256 × 256 |
| Cine TrueFISP | Coronal Oblique | Defecation | 33 | 8 | 946.4 | 1.8 | 80 | 256 × 256 |
| Cine TrueFISP | Sagittal | Postdefecation strain | 34 | 8 | 734.4 | 1.8 | 80 | 256 × 256 |
MR imaging interpretation
In order to facilitate comprehensive and efficient evaluation of the pelvic floor, the authors use a data collection sheet, which is particularly helpful when a trainee or radiologist with relatively less experience is performing the initial interpretation. In addition, they use a standardized dictation template to report the examinations ( Box 1 ). Reporting templates may differ between centers depending on the practice patterns of the referrers, but they should report anatomic findings and functional findings in all 3 pelvic floor compartments. It is imperative to include positive and pertinent negative elements that are deemed necessary by referring clinicians and radiologists, in an organized format. Further details regarding suggested elements to include in the report can be found in the later discussion, “What the referring physician needs to know.”
History: []
Technique: [] mL of [] was instilled into the rectum. Multiplanar MR imaging of the pelvis was performed using static axial T1-weighted, axial, coronal, sagittal T2-weighted, as well as dynamic multiplanar cine imaging during Kegel, defecation, and maximal strain after defecation. All images were obtained with patient in [] position.
IV Contrast: None
Comparison: None
Findings:
Anatomic Evaluation: [Prior hysterectomy/other surgery] [Levator muscle symmetry/asymmetry/atrophy/focal defects] [Prior bulking agent/urethral sling/vaginal mesh/SC mesh]
Levator plate insertion: []
Last nonmobile SC/coccygeal joint: []
[] is used as posterior point of reference for the PCL in this patient
Functional Evaluation: Patient [was/was not] able to defecate during the examination. [No/minimal/significant] rectal contrast remains after defecation.
Anterior Compartment
The bladder measures [] cm × [] cm × [] cm (volume [] mL).
Bladder base location relative to the PCL:
Rest: [] cm [above/below]
Defecation/Maximal strain: [] cm [above/below]
Findings are consistent with [no/grade 1/grade 2/grade 3] cystocele.
Urethral Angle:
Rest: [] degrees; defecation/maximal strain: [] degrees
This is consistent with [no/significant] urethral hypermobility.
Middle Compartment
Vaginal length: [] cm.
[Vaginal apex/cervix] location relative to PCL:
Rest: [] cm [above/below]
Defecation/maximal strain: [] cm [above/below]
This is consistent with [no/grade 1/grade 2/grade3] [vaginal/cervical/uterine] prolapse.
H line (levator hiatus)
Rest: [] cm (normal ≤6 cm).
Defection/maximal strain: [] cm.
M line (anorectal junction location relative to PCL)
Rest: [] cm (normal ≤2 cm below PCL)
Defecation/maximal strain: [] cm
Above findings are consistent with [normal/widened] levator hiatus and [normal/low lying] anorectal junction at rest with [no abnormal/grade 1/2/3] widening and [no abnormal/grade1/2/3] descent during defecation/maximal strain.
Posterior Compartment
Anorectal/levator-anus angle
Rest: [] degrees; Kegel: [] degrees; defecation/maximal strain: [] degrees.
This is consistent with [normal/narrowed/widened] resting angle with [expected narrowing/diminished narrowing] during Kegel and [expected widening/no change/paradoxic narrowing] during defecation/maximal strain.
Rectal Intussusception:
[No rectal intussusception/intrarectal intussusception/intraanal intussusception/extraanal intussusception] [if present, provide length of intussusception] seen.
Rectocele:
Rectocele size: [] cm anteroposterior.
Rectocele location: [upper/mid/distal vagina or along entire vaginal length]
[No/grade 1/2/3] rectocele [with/without] bulge along posterior vaginal wall
[Enterocele/peritoneocele/sigmoidocele]:
Distance below PCL: [] cm.
Distance below vaginal apex along posterior vaginal wall: [] cm.
[No/grade 1/2/3] [enterocele/peritoneocele/sigmoidocele] seen.
Other: None []
Impression:
- 1.
[Anatomic findings including prior surgery or repair]
- 2.
[Anterior compartment including cystocele/urethral mobility]
- 3.
[Middle compartment including vagina/cervix/uterus]
- 4.
[Normal/widened] levator hiatus and [normal/low lying] anorectal junction at rest with [no abnormal/grade 1/2/3] widening and [no abnormal/grade1/2/3] descent during defecation/maximal strain.
- 5.
[Rectocele?]
- 6.
[Rectal intussusception?]
- 7.
[Enterocele/peritoneocele/sigmoidocele?]
- 8.
[Normal/narrowed/widened] resting angle with [expected narrowing/diminished narrowing] during Kegel and [expected widening/no change/paradoxic narrowing] during defecation/maximal strain.
- 1.
Anatomic Evaluation
As mentioned previously, one of the advantages of dynamic MR imaging of the pelvic floor is that it also allows for high-resolution anatomic evaluation in multiple planes. Morphologic changes of the pelvic floor seen on MR imaging correlate with functional deficiencies, and there are significant differences in levator muscle volume, shape, and integrity between patients with incontinence or pelvic organ prolapse and asymptomatic individuals. The levator muscles can be assessed for areas of asymmetric thickening or atrophy, focal defects, scarring (see Fig. 3 ), ballooning ( Fig. 5 ), or focal eventration. The inferior-most levator ani muscle, the puborectalis, may be thinner on the right than on the left when viewed in the axial plane, even in asymptomatic women, likely due to chemical shift artifact. Lateral scarring or absence of the anterior attachment of the puborectalis to the pubic bone as seen on axial images may represent a tear (see Fig. 3 B). Puborectalis muscle tears may be unilateral or bilateral, and they may result from vaginal trauma or injury during childbirth, episiotomy, or other vaginal surgery. When bilateral, these may result in a “batwing shape” of the perineum at the level of the lower vagina and urethra due to absence of the pubovisceralis portion of the levator ani. The vagina may appear flat and protrude laterally into the muscle defects and lie close to the obturator internus muscle on the affected side (see Fig. 3 B). In addition to the puborectalis muscle, the anal sphincter complex should also be evaluated on axial images. The thick circular internal anal sphincter is typically intermediate in signal intensity on T2w images, whereas the more inferiorly located external sphincter is thinner and more hypointense. The levator muscles should also be evaluated for asymmetric defects, thinning, or bulging on the coronal images. These may correlate with findings of asymmetric prolapse on functional images. Sagittal images are useful to evaluate the integrity of the levator plate, which inserts on the coccygeal joints. The levator plate insertion may span multiple levels upon the coccyx; however, the dominant point of insertion should be noted.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree