MR imaging is the modality of choice for accurate local staging of bladder cancer. In addition, bladder MR imaging helps detect lymph node involvement, and in conjunction with computed tomography, provides complete staging. Familiarity with optimal imaging protocols, normal urinary bladder anatomy, and pathologic MR imaging appearances is essential for the radiologist. Evolving techniques, such as use of diffusion-weighted imaging and lymphotropic nanoparticle-enhanced MR imaging, may further enhance the ability of MR imaging in local and nodal staging.
Key points
- •
Bladder cancer continues to cause significant mortality and morbidity worldwide.
- •
MR imaging is the imaging modality of choice for accurate local staging, which is fundamental in determining further clinical management, particularly for those with T2 or greater disease.
- •
Novel MR imaging techniques, such as lymphotropic nanoparticle-enhanced MR imaging and the expanded use of diffusion-weighted imaging, may help further in local and nodal staging of disease.
Bladder cancer continues to remain a cause of more than 100,000 deaths annually worldwide. It is estimated that in the United States alone, 72,570 men and women were diagnosed with bladder cancer in 2013, with an estimated annual death rate of 15,210.
Accurate preoperative staging of bladder cancer is of paramount importance in determining the further management pathway. Radiologic and pathologic staging at initial presentation determines this, and prognosis also depends on this initial staging.
Despite involvement of ionizing radiation, computed tomography (CT) has been shown to be a valuable imaging modality in staging bladder cancer. However, MR imaging is more useful in the local staging of bladder cancer because of its inherent soft tissue resolution, soft tissue contrast, and multiplanar capabilities. Because of these combined parameters, clear differentiation between bladder wall layers is possible, therefore allowing for more accurate local staging by differentiating muscle invasive from non–muscle invasive disease, and also extramural invasion. These factors all affect further management and prognosis.
Biology of bladder carcinoma
More than 90% of bladder carcinomas are transitional cell carcinomas derived from the urothelium. About 6% to 8% are squamous cell carcinomas, and 2% are adenocarcinomas. Adenocarcinomas may be either of urachal origin or of nonurachal origin; the latter type is generally thought to arise from metaplasia of chronically irritated transitional epithelium. Pathologic grade, which is based on cellular atypia, nuclear abnormalities, and the number of mitotic figures, is of great prognostic importance.
Several etiologic factors are associated with the development of bladder cancer, but in industrialized countries, cigarette smoking is the most important. Specific chemicals have also been identified as causing bladder cancer, as have several occupational exposures to less well-defined agents including aniline dyes. Treatment with cytostatic drugs, especially cyclophosphamide, is associated with increased risk of bladder cancer, as is treatment with radiotherapy for uterine cancer. In developing countries, especially in the Middle East and parts of Africa, infections with members of the genus Schistosoma are responsible for a high incidence of bladder cancer, 75% of which are squamous cell carcinomas. Other risk factors for squamous cell carcinomas include long-term catheterization, nonfunctioning bladder (urinary stasis), and urinary tract calculi.
Muscle-invasive bladder tumors are characterized by defects in the p53 and retinoblastoma tumor suppressor genes, whereas non–muscle-invasive bladder tumors are characterized by activating mutations in the HRAS gene and fibroblast growth factor.
Biology of bladder carcinoma
More than 90% of bladder carcinomas are transitional cell carcinomas derived from the urothelium. About 6% to 8% are squamous cell carcinomas, and 2% are adenocarcinomas. Adenocarcinomas may be either of urachal origin or of nonurachal origin; the latter type is generally thought to arise from metaplasia of chronically irritated transitional epithelium. Pathologic grade, which is based on cellular atypia, nuclear abnormalities, and the number of mitotic figures, is of great prognostic importance.
Several etiologic factors are associated with the development of bladder cancer, but in industrialized countries, cigarette smoking is the most important. Specific chemicals have also been identified as causing bladder cancer, as have several occupational exposures to less well-defined agents including aniline dyes. Treatment with cytostatic drugs, especially cyclophosphamide, is associated with increased risk of bladder cancer, as is treatment with radiotherapy for uterine cancer. In developing countries, especially in the Middle East and parts of Africa, infections with members of the genus Schistosoma are responsible for a high incidence of bladder cancer, 75% of which are squamous cell carcinomas. Other risk factors for squamous cell carcinomas include long-term catheterization, nonfunctioning bladder (urinary stasis), and urinary tract calculi.
Muscle-invasive bladder tumors are characterized by defects in the p53 and retinoblastoma tumor suppressor genes, whereas non–muscle-invasive bladder tumors are characterized by activating mutations in the HRAS gene and fibroblast growth factor.
Anatomy of the urinary bladder
The urinary bladder is a musculomembranous sac, predominantly extraperitoneal, its size position and relations varying according to the amount of fluid it contains. Peritoneum covers the superior surface, or dome of the bladder. The bladder receives both ureters posterolaterally, whereas inferiorly, the bladder neck is continuous with the urethra. The orifices of the ureters at the ureterovesical junction are joined by an elevated ridge covered by mucosa (the interureteric ridge). The trigone describes a triangular region on the internal face of the bladder on the inferior wall, marked at its corners by the ureterovesical junction and the urethra.
The bladder is composed of four layers from inside out: (1) the urothelium (mucosa), (2) the lamina propria (submucosa), (3) the muscularis propria, (4) and the serosa (derived from peritoneum). The tunica mucosa is thin and smooth, continuous, above through the ureters with the lining membrane of the renal tubules, and below with that of the urethra. The thickness of the highly vascular lamina propria varies with the degree of distention of the bladder. The muscularis propria, also known as the detrusor, consists of three layers of unstriated muscular fibers: an external, middle, and an internal layer, although radiologically these are not differentiated. The serosa invests the superior surface and the upper parts of the lateral surfaces. It is reflected from these onto the abdominal and pelvic walls.
Staging of bladder cancer
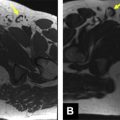
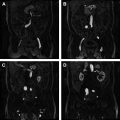
Staging of bladder cancer is based on the TNM (tumor-node-metastasis) staging system, with T stage representing the degree of bladder wall invasion ( Fig. 1 , Table 1 ). Transurethral resection of bladder tumor is often used for T1 disease, whereas partial or total cystectomy or adjuvant therapies are used for stage T2 and beyond, because an adverse side effect of transurethral resection of invasive bladder tumors is local tumor recurrence. Preoperative radiologic distinction between T1 and T2 (or greater) staging of tumors is therefore fundamental to guiding management decisions ( Fig. 2 ).
| TNM Guidelines for the Staging of Urinary Bladder Cancer | |
|---|---|
| Descriptor | Definition |
| Tumor | |
| Tx | Primary tumor cannot be evaluated |
| T0 | No primary tumor |
| Ta | Noninvasive papillary carcinoma |
| Tis | Carcinoma in situ |
| T1 | Tumor invades connective tissue under the epithelium (surface layer) |
| T2 | Tumor invades muscle |
| T2a | Superficial muscle affected (inner half) |
| T2b | Deep muscle affected (outer half) |
| T3 | Tumor invades perivesical fat |
| T3a | Tumor is detected microscopically |
| T3b | Extravesical tumor is visible macroscopically |
| T4 | Tumor invades the prostate gland, uterus, vagina, pelvic wall, or abdominal wall |
| Node | |
| Nx | Regional lymph nodes cannot be evaluated |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single lymph node <2 cm in size |
| N2 | Metastasis in a single lymph node >2 cm but <5 cm in size, or multiple lymph nodes <5 cm in size |
| N3 | Metastasis in a lymph node >5 cm in size |
| Metastasis | |
| Mx | Distant metastasis cannot be evaluated |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree