Vulvar and vaginal cancer are uncommon gynecologic malignancies, most frequently diagnosed clinically. MR imaging is a powerful tool for local staging of these tumors and to detect posttreatment complications and recurrent disease. This review presents anatomic delineation of the female pelvis, pathology and staging of vulvar and vaginal cancer, MR imaging techniques of the pelvis, MR features of vulvar and vaginal cancer, and the differential diagnosis of potential mimickers.
Key points
- •
Vulvar and vaginal cancer are uncommon gynecologic malignancies most commonly diagnosed on physical examination and pelvic biopsy.
- •
MR imaging provides excellent spatial and contrast resolution to locally stage these tumors, and detect posttreatment recurrence or complications.
- •
Although staging by the International Federation of Gynecology and Obstetrics is performed clinically, MR can assess for subtle involvement of adjacent organs and the pelvic sidewall.
- •
Optimizing the MR imaging protocol and technique is critical for optimal staging, particularly the use of endovaginal gel to distend the vaginal vault.
- •
Signal intensity, diffusion restriction, and enhancement are key imaging findings to detect and accurately stage vulvar and vaginal cancer.
Introduction
Primary vulvar cancer is an uncommon malignancy, representing 5% to 8% of all gynecologic malignancies in the United States. Primary vaginal cancer is even rarer, accounting for only 2% to 3% of gynecologic malignancies, with secondary involvement including metastatic disease or direct extension of extravaginal tumors being far more frequent. Human papillomavirus, particularly subtypes 16 and 18, has been implicated as a causative agent in both types of malignancy, resulting in an increasing incidence in the younger population. Although infrequent, late-stage vulvar and vaginal cancers and the impacts of their treatment may result in disturbing physical and emotional consequences for patients, ranging from physical disfigurement to incontinence and sexual dysfunction. The American Cancer Society estimates that nearly 6000 women will be diagnosed with vulvar cancer and 4600 women will be diagnosed with vaginal cancer in 2016.
Both vulvar and vaginal cancer are most frequently diagnosed with physical examination and pelvic biopsy. Vulvar cancer may present as a palpable vulvar mass often with pruritus, bleeding, and urinary symptoms, although it can be misdiagnosed as benign vulvar disease. Vaginal cancer usually results in painless vaginal bleeding and discharge. Although many vulvar and vaginal entities can be imaged by ultrasound and computed tomography (CT), MR imaging is the modality of choice to image complex vaginal and vulvar anatomy and pathology. Due to its superlative soft tissue contrast and high-resolution multiplanar imaging without ionizing radiation, MR is a superb modality for local staging of vaginal and vulvar cancers and surveillance after treatment. Combined PET/MR imaging offers potential in the staging and surveillance in gynecologic malignancies, and future studies will likely demonstrate value in assessment of vulvar and vaginal carcinomas.
This review focuses on anatomy of the vulva and vagina as depicted on MR imaging, the pathology of vulvar and vaginal carcinomas, MR imaging techniques for the female pelvis, MR imaging features of vulvar and vaginal cancers, and the differential diagnosis of these lesions and pitfalls in diagnosis.
Introduction
Primary vulvar cancer is an uncommon malignancy, representing 5% to 8% of all gynecologic malignancies in the United States. Primary vaginal cancer is even rarer, accounting for only 2% to 3% of gynecologic malignancies, with secondary involvement including metastatic disease or direct extension of extravaginal tumors being far more frequent. Human papillomavirus, particularly subtypes 16 and 18, has been implicated as a causative agent in both types of malignancy, resulting in an increasing incidence in the younger population. Although infrequent, late-stage vulvar and vaginal cancers and the impacts of their treatment may result in disturbing physical and emotional consequences for patients, ranging from physical disfigurement to incontinence and sexual dysfunction. The American Cancer Society estimates that nearly 6000 women will be diagnosed with vulvar cancer and 4600 women will be diagnosed with vaginal cancer in 2016.
Both vulvar and vaginal cancer are most frequently diagnosed with physical examination and pelvic biopsy. Vulvar cancer may present as a palpable vulvar mass often with pruritus, bleeding, and urinary symptoms, although it can be misdiagnosed as benign vulvar disease. Vaginal cancer usually results in painless vaginal bleeding and discharge. Although many vulvar and vaginal entities can be imaged by ultrasound and computed tomography (CT), MR imaging is the modality of choice to image complex vaginal and vulvar anatomy and pathology. Due to its superlative soft tissue contrast and high-resolution multiplanar imaging without ionizing radiation, MR is a superb modality for local staging of vaginal and vulvar cancers and surveillance after treatment. Combined PET/MR imaging offers potential in the staging and surveillance in gynecologic malignancies, and future studies will likely demonstrate value in assessment of vulvar and vaginal carcinomas.
This review focuses on anatomy of the vulva and vagina as depicted on MR imaging, the pathology of vulvar and vaginal carcinomas, MR imaging techniques for the female pelvis, MR imaging features of vulvar and vaginal cancers, and the differential diagnosis of these lesions and pitfalls in diagnosis.
Anatomy
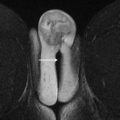
The urogenital triangle of the female perineum is defined by the pubic symphysis anteriorly, an imaginary line between the ischial tuberosities posteriorly, and the ischiopubic rami anterolaterally. The vulva is the triangular shaped structure of the female external genitalia, bounded superficially by the skin and deeply by the urogenital diaphragm, and consisting of the labia majora laterally and labia minora medially ( Fig. 1 ). The vestibule surrounds the external urethral meatus and vaginal introitus, with the Bartholin glands positioned posterolateral on each side of the introitus that secrete lubricants into the vestibule.
The vagina is a fibromuscular tube that extends from the introitus to the cervix ( Fig. 2 ). The vaginal mucosa is T2 hyperintense, blending with vaginal secretions and any gel used to distend the vaginal cavity; in contrast to the T2 hypointense submucosal and muscularis layers. The adventitial layer of the vagina contains the vaginal venous plexus, which is T2 hyperintense and avidly enhances after intravenous gadolinium administration.
Lymphatic drainage of the vagina varies depending on the segment of vagina in question. Drainage of the upper third of the vagina follows that of the uterine vessels, draining to obturator, internal iliac, and external iliac lymph nodes, followed by the para-aortic lymph nodes. Drainage of the lower third of the vagina follows that of vaginal vessels, draining to inguinal, femoral, and, less frequently, the mesorectal lymph nodes. The middle third of the vagina may drain via either or both routes. Sentinel lymph node mapping is often performed before surgery due to variability in this drainage pattern.
Lymphatic drainage of the vulva is primarily through the superficial inguinal lymph nodes. The superficial inguinal lymph nodes can be subdivided into 3 groups: a medial group, medial to the femoral vein and greater saphenous vein; an intermediate group near the femoral and saphenous vein; and a lateral group in the lateral third of the groin. The deep inguinal (also referred to as femoral) lymph nodes are located medial to the femoral vein and drain to the external iliac lymph nodes.
Pathology and staging
Vulvar carcinoma is squamous cell in origin in 90% of cases. Melanoma, basal cell carcinoma, Paget disease, Bartholin gland cancer, and adenocarcinoma are much rarer forms of vulvar malignancies. Human papillomavirus (HPV)-associated vulvar squamous cell carcinoma (SCC) presents in women younger than 60 years, is associated with vulvar intraepithelial neoplasia, and may be multifocal with associated vaginal and cervical tumors. Non–HPV-associated vulvar SCC presents in older women, is associated with lichen sclerosis or vulvar inflammation, and is more typically unifocal. The most recent revision of the International Federation of Gynecology and Obstetrics (FIGO) staging system for vulvar cancer from 2009 guides the clinicopathologic staging of vulvar cancer.
| FIGO Stage | Features |
|---|---|
| IA | Tumor <2 cm with <1 mm stromal invasion, confined to vulva or perineum |
| IB | Tumor >2 cm or with >1 mm stromal invasion, confined to vulva or perineum |
| II | Any size tumor extending to adjacent perineal structures (distal third of the urethra or vagina, anus), no positive nodes |
| IIIA | Any size tumor with positive lymph nodes (1–2 <5 mm or 1 >5 mm) |
| IIIB | Any size tumor with positive lymph nodes (3+ <5 mm or 2+ >5 mm) |
| IIIC | Any size tumor with positive nodes and extracapsular spread |
| IVA | Tumor invading upper two-thirds of urethra or vagina, bladder, rectum, or fixed to pelvic bone; or fixed or ulcerated regional positive lymph nodes |
| IVB | Distant metastases, including pelvic lymph nodes |
Similarly, vaginal cancer is overwhelmingly squamous cell in origin, representing 80% to 90% of cases. Clear-cell adenocarcinoma, resulting from in utero exposure to diethylstilbestrol (DES), is rarely seen today due to cessation of the use of DES in pregnancy, but previously represented 4% to 10% of vaginal tumors and developed in young women. Other less frequent histologic subtypes of vaginal malignancies include melanoma, sarcoma, carcinoid, and small-cell neuroendocrine. Like vulvar cancer, HPV has been implicated in the development of vaginal SCC. Vaginal cancer is staged clinically according to the 2009 FIGO staging system, as many patients will not undergo surgical management.
| FIGO Stage | Features |
|---|---|
| I | Tumor limited to vaginal wall |
| II | Tumor involves paravaginal tissue without extension to pelvic side wall |
| III | Tumor extends to pelvic side wall |
| IVA | Tumor extends beyond true pelvis, or involves bladder and rectal mucosa |
| IVB | Distant metastases |
MR Imaging Protocol
Pelvic MR protocols used for staging and surveillance in vulvar or vaginal tumors may vary from one institution to the next. At the Mallinckrodt Institute of Radiology, the patient is asked to void before entering the MR imaging suite. The patient is then asked to self-administer 20 mL of a water-soluble gel into the vaginal vault before beginning the MR examination. A pelvic or torso phased array coil is used for the study. The examination begins with acquisition of localizer imaging and a coronal breath-hold T2-weighted single-shot turbo spin-echo sequence (SSTSE) covering the entire pelvic cavity, with sufficient cranial extent to visualize the kidneys (specifically to assess for hydronephrosis). The initial precontrast sequences include a series of multiplanar high-resolution TSE T2-weighted imaging of the entire pelvis and axial small field of view T2-weighted images of the female reproductive system, gradient-recalled echo (GRE) T1-weighted in-phase and out-of-phase axial chemical shift imaging (a routine component of our female pelvic MR protocols), echo planar diffusion-weighted axial imaging with b values of 50, 500, and 1000 (with a calculated apparent diffusion coefficient [ADC] map), and 3-dimensional (3D) spoiled gradient-echo fat-suppressed T1-weighted axial imaging of the pelvis. An extracellular gadolinium contrast agent is administered at a dose of 0.1 mmol/kg at an injection rate of 2 mL/s. After an 18-second delay, dynamic T1 spoiled gradient-echo fat-suppressed axial imaging is performed at 15-second intervals for 3 scans, to best achieve arterial, venous, and equilibrium phase images; followed by sagittal and coronal oblique post–contrast-enhanced images.
| Sequence | TR | TE | FOV | Matrix | Slice Thickness, mm | NSA |
|---|---|---|---|---|---|---|
| T2W coronal SSTSE | 1000 | 81 | 400 × 400 | 320 × 320 | 8 | 1 |
| T2W axial TSE | 8240 | 115 | 350 × 260 | 448 × 512 | 6 | 2 |
| T1W axial GRE on and out-of-phase | 120 | 2.38 | 350 × 260 | 240 × 320 | 8 | 1 |
| EPI axial DWI (b = 50, 500, 1000) | 13,100 | 91 | 350 × 260 | 112 × 128 | 3 | 2 |
| T2W sagittal TSE | 5030 | 125 | 280 × 320 | 512 × 448 | 5 | 2 |
| T2W axial TSE small FOV | 5770 | 115 | 220 × 250 | 512 × 448 | 5 | 2 |
| T2W coronal TSE | 5500 | 115 | 350 × 350 | 512 × 512 | 6 | 2 |
| T1W 3D FS spoiled GRE axial | 4.66 | 2.38 | 350 × 260 | 540 × 640 | 3.5 | 1 |
| Administer 0.1 mmol/kg intravenous gadolinium contrast at 2 mL/s, and wait 18 s | ||||||
| T1W 3D FS spoiled GRE axial ×3 | 4.66 | 2.38 | 350 × 260 | 540 × 640 | 3.5 | 1 |
| T1W 3D FS spoiled GRE sagittal | 4.66 | 2.38 | 350 × 350 | 640 × 640 | 3.5 | 1 |
| T1W 3D FS spoiled GRE coronal | 4.66 | 2.38 | 410 × 410 | 540 × 640 | 3.5 | 1 |
MR imaging findings
Vulvar Cancer
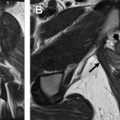
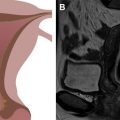
Vulvar cancer manifests on MR imaging as a T1 hypointense to isointense, T2 intermediately hyperintense (“evil gray”), solidly enhancing mass with associated diffusion restriction ( Figs. 3–6 ). Diffusion-weighted imaging may aid in tumor detection with better tumor-to-normal tissue contrast than T2-weighted imaging. The use of fat suppression with contrast-enhanced imaging improves lesion conspicuity. As the presence of a vulvar mass is rarely in doubt when patients are referred for pelvic MR imaging, the emphasis is on defining the anatomic extent of the tumor. Involvement of superficial structures, such as the labia minora and majora, urethra, and clitoris, and deeper organs, such as the vagina and anus by tumor, as well as size of the tumor, should be assessed ( Fig. 7 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree