There are many ovarian cancer subtypes, giving rise to a range of appearances at gross pathology and magnetic resonance (MR) imaging. Certain fundamental concepts at MR, arising from underlying tissue characteristics, can provide guidance to radiologists in suggesting a diagnosis. The ability of multiparametric MR to risk stratify ovarian masses can contribute substantially to clinical decision making and patient management.
Key points
- •
Multiparametric MR imaging can assist in differentiating subtypes of ovarian cancer.
- •
Very low T2-weighted signal suggests low-grade or benign disorder.
- •
Macroscopic fat in a lesion suggests mature teratoma as the diagnosis.
- •
High-grade serous carcinoma of the ovary arises from a precursor in the fallopian tube mucosa, referred to as serous tubal intraepithelial carcinoma.
Introduction
The normal ovary contains epithelial, germ cell, and mesenchymal elements. Each of these cell types can undergo neoplasia, resulting in a wide variety of possible tumors arising from a single organ. More than 30 subtypes of ovarian neoplasm have been described. However, most are in one of 3 major categories: epithelial, germ cell, or stromal neoplasms. Some cell types give rise to benign and malignant neoplasms. This article primarily focuses on malignant neoplasms, with some discussion devoted to low-grade and benign ovarian masses.
Ovarian cancer may have nonspecific, subtle, or absent symptoms, and many lesions are detected incidentally. Thus, it is important to recognize concerning imaging features in all types of imaging studies. Ultrasonography has a primary role in adnexal mass detection, whereas MR imaging enables characterization of sonographically indeterminate masses. Computed tomography (CT) and PET/CT do not have a primary role in lesion characterization, but are important for staging and follow-up of known ovarian cancer.
With advances in spatial and contrast resolution, and development of functional imaging techniques including perfusion and diffusion, MR imaging has increasing capacity to distinguish benign from malignant adnexal lesions. This article focuses on MR imaging findings concerning for neoplasm, the gross and microscopic features differentiating the subtypes of ovarian malignancy, and correlation between these.
Introduction
The normal ovary contains epithelial, germ cell, and mesenchymal elements. Each of these cell types can undergo neoplasia, resulting in a wide variety of possible tumors arising from a single organ. More than 30 subtypes of ovarian neoplasm have been described. However, most are in one of 3 major categories: epithelial, germ cell, or stromal neoplasms. Some cell types give rise to benign and malignant neoplasms. This article primarily focuses on malignant neoplasms, with some discussion devoted to low-grade and benign ovarian masses.
Ovarian cancer may have nonspecific, subtle, or absent symptoms, and many lesions are detected incidentally. Thus, it is important to recognize concerning imaging features in all types of imaging studies. Ultrasonography has a primary role in adnexal mass detection, whereas MR imaging enables characterization of sonographically indeterminate masses. Computed tomography (CT) and PET/CT do not have a primary role in lesion characterization, but are important for staging and follow-up of known ovarian cancer.
With advances in spatial and contrast resolution, and development of functional imaging techniques including perfusion and diffusion, MR imaging has increasing capacity to distinguish benign from malignant adnexal lesions. This article focuses on MR imaging findings concerning for neoplasm, the gross and microscopic features differentiating the subtypes of ovarian malignancy, and correlation between these.
Magnetic resonance protocol for adnexal mass characterization
Imaging parameters at different institutions vary according to equipment and local preference, but the authors consider the following pulse sequences to be important in the evaluation of a suspected ovarian mass. Before imaging, glucagon or another antiperistaltic agent is beneficial in reducing artifact from bowel and uterine peristalsis.
- •
T1-weighted (T1W) images in the axial plane are obtained without and with fat saturation, to identify foci of macroscopic fat and differentiate them from hemorrhage. T1W chemical shift imaging using dual in-phase and opposed-phase gradient echo (GRE) sequences in the axial plane are useful for confirming the presence of intralesional lipid and macroscopic fat.
- •
T2-weighted (T2W) images in the axial, sagittal, and coronal planes are obtained without fat saturation. Multiplanar T2W imaging (T2WI) is essential for optimizing tissue characterization and differentiating solid from cystic components. Alternatively, high-resolution single-plane T2WI can be performed as a three-dimensional acquisition, lengthening scan time but enabling isotropic multiplanar reformats. As discussed later, there is a small subset of adnexal masses that are intrinsically low signal on T2WI, which helps narrow the differential diagnosis.
- •
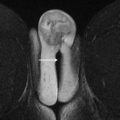
Multiphase or dynamic contrast-enhanced T1W images using gadolinium-based intravenous contrast agents are critical to evaluate vascularity of any soft tissue elements, including papillary projections, masses and nodules, or thick septations. Postcontrast imaging includes fat-saturated multiphase image acquisitions before contrast, during the arterial and venous phases, and in 1 or more delayed phases. Precontrast and postcontrast imaging should be performed with identical coverage and scan parameters to enable image subtraction, which is of particular importance in hemorrhagic lesions in which intrinsic hyperintensity on T1W imaging (T1WI) can mimic enhancement ( Fig. 1 ). In some centers, a true dynamic acquisition through the area of interest is performed, using multiple short acquisitions (15 seconds) repeated over a period of 3 to 4 minutes and enabling visual and quantitative analysis of enhancement kinetics.
Fig. 1
A 74-year-old woman with long-standing endometrioma. MR imaging was ordered to evaluate for any evidence of neoplastic transformation. ( A ) Precontrast T1WI plus fat saturation (FS) shows a lobulated, very-high-signal right adnexal lesion ( arrow ) containing hemorrhagic products. ( B ) This area remains high signal ( arrow ) on postcontrast T1WI + FS and enhancement is difficult to evaluate. ( C ) Subtraction imaging shows a matched low-signal area ( arrow ), indicating lack of enhancement.
- •
Diffusion-weighted imaging (DWI) performed at both low and high b values (>b 800 ) has some utility for characterization of adnexal masses, and is especially useful in the detection of drop metastases and peritoneal implants. Both omental cake and peritoneal deposits often retain high signal intensity at high b values, increasing the conspicuity of metastases. Lymph nodes can also be more easily detected with the assistance of high-b-value DWI. Regarding the primary mass, there is substantial overlap in apparent diffusion coefficient (ADC) values between malignant and highly cellular benign lesions, with false-positives limiting the utility of quantitative comparison. However, there are some specific areas of utility. Fibrous masses with very low ADC values usually also show low signal on DWI, suggesting benignity. Further, masses with benign features on both ADC maps and DWI are most often benign, and demonstration of low DWI signal within a mass may augment diagnostic confidence when features on other sequences favor a benign process.
Epithelial ovarian tumors
Ovarian cancer is the fifth leading cause of cancer death in women, and most ovarian neoplasms are epithelial in origin. Serous and mucinous histologies are the predominant epithelial subtypes, with serous carcinomas being the most common (60%) and associated with the highest mortality.
Epithelial ovarian tumors can also be grouped into 2 broad categories based on genetic lineage: type I and type II. Type I tumors include low-grade serous carcinoma (LGSC), and low-grade endometrioid, clear cell, and mucinous carcinomas, all of which are slow growing and develop from well-established precursor lesions. In general, type I tumors present as large masses confined to the ovary, have an indolent course, and have a good prognosis. In contrast, type II tumors tend to present at advanced stage with a poorer prognosis. Type II tumors include high-grade serous carcinoma (HGSC), high-grade endometrioid carcinoma, carcinosarcoma, and undifferentiated carcinomas. At a molecular level, type I tumors are fairly genetically stable, whereas type II tumors are highly unstable, with p53 mutations present in greater than 95% of cases.
Serous Cystadenoma, Borderline Tumor, and Adenocarcinoma
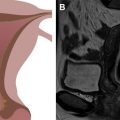
Serous cystadenoma and cystadenofibroma are strictly benign lesions. They are usually smooth walled and unilocular with minimal papillary excrescences ( Fig. 2 ). Serous borderline tumor, LGSC, and HGSC have increasing degrees of cytologic atypia, and, particularly in the case of HGSC, mitotic activity. The serous cells constituting each subtype closely resemble fallopian tube epithelium, and can contain numerous ciliated cells in cases of cystadenoma/cystadenofibroma, serous borderline tumor, and LGSC. Most frankly malignant serous tumors involve both ovaries pathologically, although 1 or both may be normal in size by imaging. These cancers are typically mixed cystic and solid, and the amount and complexity of the solid tissue correlates with risk of malignancy. Classic papillary architecture and psammomatous calcifications can be appreciated by imaging and under the microscope. These 3 subtypes are discussed later.
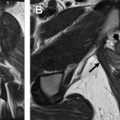
Serous borderline tumors are thought to develop from neoplastic transformation of serous cystadenomas, usually through acquisition of either BRAF or KRAS mutations. In general, these tumors are characterized by slow growth with overall excellent prognosis. These tumors usually form cysts containing intracystic excrescences that resemble broad-based papillary buds. These buds are often branched and are composed of fibrotic stroma lined by serous epithelium, subsets of which are ciliated. They can also form fine papillary soft tissue elements within cysts not supported by stromal buds. The serous epithelial cells may invade into ovarian stroma; however, the invasive areas are small and focal by definition (<5 mm in greatest dimension). Although it was originally introduced as a provisional category between cystadenoma and carcinoma, “borderline” is now a formal pathologic designation rather than an indication of uncertainty. Borderline serous carcinoma can manifest with peritoneal implants ( Fig. 3 ) and metastatic lymphadenopathy in 35% and 27% of patients respectively. Regardless, the 10-year survival of patients with serous borderline tumor remains high at 96% to 100%.
The pathogenesis of LGSC is also unclear but cases may develop from serous borderline tumors. These tumors share similar pathologic and molecular features (eg, KRAS mutations) with serous borderline tumors, and tend to have a better prognosis than HGSC given their indolent growth, possibly secondary to lack of p53 mutations. LGSC are well differentiated, frequently contain calcifications, and maintain a cystic and papillary architecture with little necrosis, evident both on MR imaging and gross pathologic examination. At imaging, these tumors are often large, complex, cystic masses with well-marginated septa and solid components, potentially distinguishable from HGSCs when contralateral masses, ascites, omental caking, and lymphadenopathy are absent.
Terminology regarding LGSC has historically been confusing because cases can closely resemble borderline tumors, and thus pathologic discrimination can be difficult. In general, LGSC is reserved for cases in which the primary tumor shows definitive stromal invasion (≥5 mm). However, noninvasive forms of LGSC, characterized by areas of confluent epithelial growth (≥5 mm) but without stromal invasion, are also recognized by gynecologic pathologists. Pathologic terminology in these cases is especially confusing because the terms noninvasive LGSC and serous borderline tumor, micropapillary/cribriform variant, are regarded as equivalent. In addition, transformation of LGSC to HGSC is a very rare phenomenon.
HGSC are type II tumors frequently associated with early p53 mutations. Although most BRCA-related hereditary ovarian cancers are HGSCs, BRCA mutation carriers have a better prognosis than women with sporadic ovarian HGSCs. In general, these are very aggressive tumors that often present at advanced stage with overall poor prognosis. It is now well established that HGSC of the ovary arises from a precursor in the fallopian tube mucosa, or at least tubal-type epithelium, rather than from low-grade lesions of the ovary itself. The precursor lesion in the fallopian tube is referred to as serous tubal intraepithelial carcinoma (STIC). Further supporting the connection, STIC is absent in nonserous ovarian carcinomas, such as mucinous and endometrioid types. At imaging, HGSCs often appear as complex cystic masses with solid components ( Fig. 4 ), but some are completely solid. There is rapid intracoelomic spread, with peritoneal and multiorgan surface involvement sometimes even in the context of normal-sized or minimally enlarged ovaries. The origin of HGSC in the fallopian tube could help explain the high incidence of peritoneal disease at time of diagnosis.
Mucinous Cystadenoma, Borderline Tumor, and Adenocarcinoma
In contrast with the bimodal lineage of serous tumors, ovarian mucinous neoplasms probably progress sequentially from cystadenoma, to borderline, to carcinoma. This process has been theorized given the presence of adenoma, borderline, and carcinomatous components in cases of mucinous carcinoma, as well as identical KRAS mutations in each component. Overall, mucinous neoplasms are the second most common ovarian epithelial neoplasm. On histology, most mucinous neoplasms are benign or borderline (80%), with carcinoma representing a small fraction. In contrast with serous tumors, which are commonly bilateral, most ovarian mucinous tumors are unilateral. These tumors may attain a very large size while remaining benign.
Mucinous cystadenoma is predominantly cystic at imaging, unilocular, multilocular, or with multiple thin, smooth septations ( Fig. 5 ). On histology, the epithelial cells lining the cysts comprise a population of well-differentiated mucinous cells arranged in a simple, nonstratified pattern. There is some variation in the appearance in the epithelial cells, with some resembling colorectal mucosa (including goblet cells), small intestine (sometimes featuring Paneth cells), or upper gastrointestinal pyloric mucosa. It had been thought that ovarian mucinous cystadenomas could also contain endocervical-type (müllerian) mucinous epithelium, but it is likely that these are mucinous cells of an upper gastrointestinal phenotype mimicking endocervical epithelium. Seromucinous ovarian neoplasms are likely to be fundamentally different from mucinous cystadenomas, because the former often arise in a background of endometriosis, which is an uncommon phenomenon in ovarian mucinous tumors. Mucinous borderline tumors are usually large, with smooth capsules and no grossly evident papillations/excrescences. As such, they are likely to be indistinguishable from mucinous cystadenoma by imaging, because both tumors may be multicystic and large. Microscopically, mucinous borderline tumors are also composed of gastrointestinal-type epithelium, usually with a colorectal or intestinal appearance. The pathologic diagnosis of mucinous borderline tumor is rendered when proliferative areas comprise greater than 10% of the epithelial volume ; compared with mucinous cystadenoma, borderline tumors have a greater amount of the epithelial component, which manifests as closely packed cells with architectural complexity (microscopic tufting). Similarly, mucinous borderline tumors also tend to show a greater degree of cytologic atypia compared with cystadenomas. Mucinous borderline tumors can show striking nuclear atypia to the point at which there is intraepithelial carcinoma (mucinous borderline tumor with intraepithelial carcinoma), and microinvasion may also be present (by definition, the invasive foci must be small, measuring <5 mm in greatest linear extent). The long-term disease-related survival nears 100%, even in patients with intraepithelial carcinoma, because older descriptions of so-called invasive mucinous borderline tumors are now thought to most likely represent metastases from the gastrointestinal tract. There are insufficient data regarding the behavior of mucinous borderline tumors with microinvasion.
Mucinous adenocarcinoma is also cystic, but shows thick, nodular septa. Historically, the prevalence of invasive mucinous tumors was significantly overestimated, because mucinous gastrointestinal metastases to the ovary are now known to be much more common than primary mucinous ovarian carcinoma. Classically, metastases are usually bilateral, whereas primary invasive mucinous cancer is usually unilateral. However, metastases can present clinically as a unilateral ovarian mass with bilateral involvement only encountered microscopically. In contrast with benign mucinous tumors, cystadenocarcinoma shows frank ovarian stromal invasion microscopically. Metastatic tumors to the ovary are usually smaller than primary ovarian tumors but, similarly to laterality, the range of size in metastatic cases is broad and overlaps with primary ovarian tumors. Gross or radiographic ovarian surface involvement and presence of extraovarian disease (including clinical pseudomyxoma peritoneii [PMP]) at presentation should also prompt investigation for a nongynecologic primary. Primary ovarian mucinous adenocarcinomas are characterized by destructive stromal invasion (>5 mm) and/or expansile epithelial growth, the latter defined by confluent glands with little intervening stroma. Mucinous adenocarcinoma may also show mural nodules grossly and radiographically characterized by solid growths arising adjacent to or within cysts. Microscopically, these mural nodules often manifest as an infiltrative proliferation of poorly-differentiated carcinoma cells (anaplastic carcinoma) or cells resembling a sarcoma (sarcomalike mural nodule). All 3 patterns may exist in the same tumor, and all carry a risk for recurrence. The pattern of spread differs from serous cancer, because there is less transcoelomic growth along the peritoneal surfaces. Instead, invasive mucinous cancers tend to invade into the abdominal wall and metastasize to solid organs (liver and spleen). PMP is a clinical term for a specific type of intraperitoneal disease that is usually secondary to a nongynecologic mucinous tumor (eg, metastatic appendiceal mucinous neoplasm). Cases of PMP may manifest as abundant extracellular mucin with a minority of bland gastrointestinal-type epithelial cells, and symptoms are usually related to tumor bulk.
Endometrioid Ovarian Carcinoma
Endometrioid ovarian cancer is associated with long-standing endometriosis, and endometriomas and endometriotic cysts are probably its benign precursors. After serous carcinoma, endometrioid is the second most common type of ovarian carcinoma, although mucinous tumors (usually benign) are more common overall. The risk of malignancy is proportional to size of endometriotic deposit and patient age. In some series, endometriosis in the same ovary or elsewhere in the pelvis is observed in up to 42% of cases. Endometrioid ovarian adenocarcinoma is associated with microsatellite instability, usually via loss of MLJ1 or MLH2 expression, and can be seen in patients with Lynch syndrome.
Endometrioid neoplasms of the ovary also follow a benign, borderline, and malignant subclassification scheme. Specifically, these include endometrioid adenofibroma, atypical proliferative (borderline) endometrioid tumor, and endometrioid adenocarcinoma. Because of its clear association with endometriosis, there are various other lesions that, although not representative of outright carcinoma, are atypical enough to earn designations as atypical endometriosis. This term has been used in endometriotic cysts lined by cytologically atypical cells, as well as cases of endometriosis with features identical to those seen in atypical endometrial hyperplasia. Endometrioid adenocarcinoma is usually a smooth-walled, multiseptate cystic mass containing blood products, variable amounts of fibrotic stroma, and friable soft tissue elements on gross examination that manifest on MR imaging as enhancing intracystic soft tissue components ( Fig. 6 ). Thus, it is important to carefully assess for the presence of enhancement within all endometriomas. Microscopically, these tumors closely resemble their counterparts in the endometrium. Most of these tumors are low grade, resembling FIGO (International Federation of Gynecology and Obstetrics) grade 1 endometrial endometrioid adenocarcinoma; however, grading of endometrioid ovarian carcinoma is controversial. Although these tumors are histologically malignant, most present as early-stage unilateral tumors confined to the ovary without extraovarian disease.